National Fisheries Institute: U.S. ports lead the world in imported seafood refusals

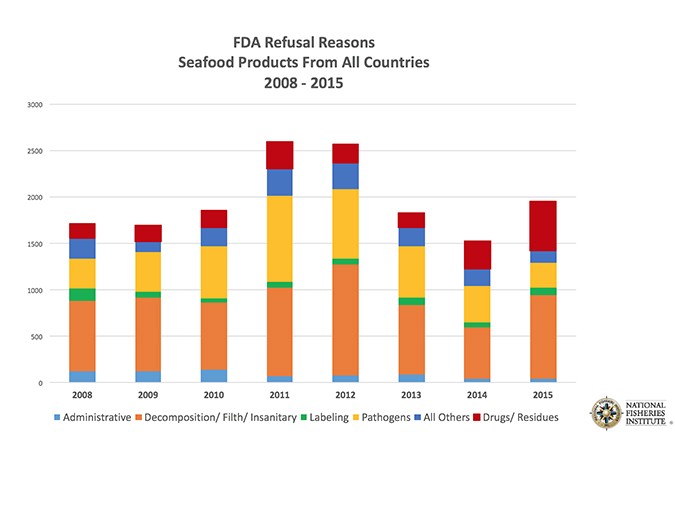
The U.S. Food and Drug Administration refused nearly 2,000 shipments of imported seafood in 2015, one of the highest totals in recent years (see Fig. 1). More than one-fourth of those refusals were because illegal substances were detected, most likely veterinary drugs administered to farmed fish and shellfish. It was the third-straight year of increased drug-related refusals.
“Over the years the refusal rate hasn’t disappeared. They’re still finding unapproved residues in seafood products,” said Lisa Weddig, VP-regulatory and technical affairs at the National Fisheries Institute (NFI), which compiled import-refusal statistics for a presentation at Seafood Expo North America (SENA) in Boston in early March.
Weddig moderated the conference session titled “Understanding How FDA Regulates Aquaculture Drugs,” during which attendees were urged to arm themselves with information about legal and illegal veterinary drugs used in aquaculture. Concern over drugs used in aquaculture presents an obstacle for a seafood industry seeking to attract more consumer interest. The precarious reputation that aquaculture has with U.S. consumers was a common discussion thread at the show.
“Farmed imported product is safe, but what does the public think of it?” Weddig asked. “We want to sell more fish and paint a [positive] picture of seafood. But it’s the Achilles heel of the industry — it’s hard to promote a product with all the negative things being said about it.”
Peter Koufopoulos, director of the Division of Seafood Safety at FDA’s Center for Food Safety and Applied Nutrition (CFSAN), said the agency urges processors and farms to work together in order to retain their ability to ship product to the United States. It’s not just about traces of substances detected in fish fillets by laboratories. Koufopoulos said unapproved veterinary drugs should not be used at any point in the production cycle, even if they are approved in other countries.
We want to sell more fish and paint a [positive] picture of seafood. But it’s the Achilles heel of the industry — it’s hard to promote a product with all the negative things being said about it.
“There is no tolerance for unapproved drugs. There should be no use whatsoever,” Koufopoulos said, before giving the audience some insight into how the agency screens for potential violations, tests products and enforces regulations. FDA officials are always looking at trends, he said.
“Once we start seeing a problem it gets flagged. Then we start seeing more. We might sample more by country, by facility, by product line. We also anticipate that it might be happening in other places,” he said. “We may expand our reach to find out how big a problem it is, once we start seeing refusals.
“When something gets flagged [sampling and monitoring] goes up 100 percent.”
The Food, Drug and Cosmetic Act requires that food products be unadulterated, and includes provisions that deal with new drugs, both human and animal. The agency’s inspection regime requires all exporters to adhere to HACCP (Hazard Analysis Critical Control Points) regulations for food safety. Under the Seafood HACCP Regulation, processors of farmed fish are expected to understand the hazard of unapproved drugs and put in place preventive controls to ensure that drug usage is legal and appropriate.
Despite a well-publicized 2007 Associated Press report that found that just 1.3 percent of imported fish, vegetables, fruit and other foods are regularly inspected, the FDA has a robust risk-based inspection policy determined by relative likelihood and severity of potential food safety concerns.
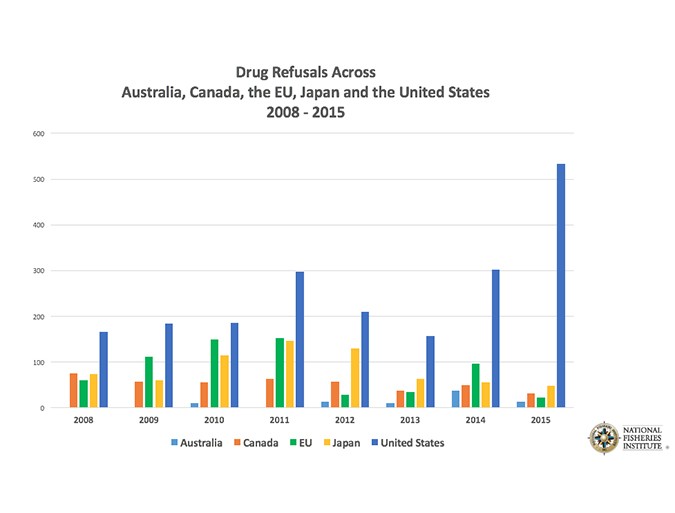
What’s more, the agency refuses more seafood shipments than many other major markets (see Fig. 2) due to the detection of illegal substances. In 2015, FDA refused 534 shipments due to illegal drugs, second only to “decomposition, filth or unsanitary” product conditions. It’s more than 10 times the number of refusals by Japan (49), one of the world’s biggest seafood importers, according to NFI’s data.
There are only a handful of drugs approved for seafood production, and must be approved for the exact species being produced:
- Chorionic gonadotropin, approved for brood finfish.
- Formalin, for finfish, finfish eggs, Penaeid shrimp, salmon, trout, catfish, largemouth bass and bluegill.
- Florfenicol, for freshwater-reared finfish.
- Tricaine methanesulfonate, for the Ictaluridae, Salmonidae, Esocidae and Percidae families.
- Oxytetracycline dihydrate, for catfish, salmonids and lobster.
- Oxytetracycline hydrochloride, for finfish fry and fingerlings.
- Hydrogen peroxide, for finfish eggs, salmonids, freshwater/coldwater finfish and channel catfish.
- Sulfamerazine, trout (rainbow, brook, brown).
- Sulfadimethoxine/ormetoprim, catfish and salmonids.
- Chloramine-T, freshwater salmonids, walleye, freshwater/warmwater finfish.
There are a number of other drugs that have been used in the past but are on the banned list and considered a high enforcement priority:
- Chloramphenicol
- Nitrofurans
- Fluoroquinolones and Quinolones
- Malachite Green
- Steroid hormones
Author
-

James Wright
Editorial Manager
Global Aquaculture Alliance
Portsmouth, NH, USA[103,114,111,46,101,99,110,97,105,108,108,97,97,103,64,116,104,103,105,114,119,46,115,101,109,97,106]
Tagged With
Related Posts

Intelligence
Risk v. hazard: A dispassionate look at pangasius
Vietnam’s pangasius industry captivated the global seafood industry, environmental organizations and the mass media. A scientific look at harmful substances detected in exported fillets and the reporting of the associated health risks through the media finds wide disparities.

Intelligence
Surmounting the consumer disconnect on farmed seafood
In his first piece for the Advocate, industry veteran Phil Walsh, VP of Growth for Alfa Gamma Seafood Group in Miami, wonders why consumers are so comfortable with farm-raised land animals, but not farm-raised fish.

Intelligence
Aquaculture 2016: Examining the industry’s role in the food system
A wide range of important topics was discussed at the Aquaculture 2016 conference and trade show in Las Vegas last week. Editor Emeritus Darryl Jory shares his notes from the four-day event, which occurs every three years.

Intelligence
GM salmon and the FDA: 10 takeaways
After a 20-year process, a genetically modified fish earned U.S. Food and Drug Administration approval, reigniting one of the seafood industry’s most intriguing controversies. Here are 10 key downloads from the groundbreaking decision over AquAdvantage salmon.

