Clinical syndromes are species-specific

The infectious hypodermal and hematopoietic necrosis virus (IHHNV) was first discovered in Pacific blue shrimp, Penaeus stylirostris, in 1981, when it caused epizootics associated with mass mortality. IHHNV is the smallest known penaeid shrimp virus. Containing single-stranded DNA with an estimated size of 4.1 kb, it is one of the most prevalent and widespread shrimp viruses. IHHNV can be transmitted both vertically and horizontally.
Once a population is infected with IHHNV, it is very difficult to eradicate the virus. Both the World Organization for Animal Health and United States Marine Shrimp Farming Program list IHHNV as a major pathogenic agent excluded from specific pathogen-free status.
Various shrimp species can be infected by IHHNV in all developmental stages. However, the clinical syndromes are species-specific. Unlike the effects shown in Pacific blue shrimp, infection of Pacific white shrimp, P. vannamei, with IHHNV does not result in mass mortality. Instead, animals show runt deformity syndrome (RDS) and large size variation at harvest. For P. stylirostris, an IHHNV refractory line was developed and successfully applied in commercial production during the late 1990s.
IHHNV study
To assess the possibility of selecting IHHNV-free shrimp from an infected population and the feasibility of developing an IHHNV refractory line for P. vannamei, the dominant shrimp species currently cultured worldwide, the authors conducted a large-scale study to investigate family effects on the prevalence of IHHNV in P. vannamei broodstock within the context of a family-based genetic selection program based on non-SPF populations.
The specific objectives were to estimate the IHHNV prevalence in the existing population, investigate the genetic variability of IHHNV susceptibility among families and evaluate the quantitative effects of IHHNV prevalence on shrimp production performance traits. Technical support for the study was provided by Linda Nunan, Solangel Navarro, Brenda Noble and Dr. Donald Lightner of the Aquaculture Pathology Laboratory at the University of Arizona in the United States.
Experimental design
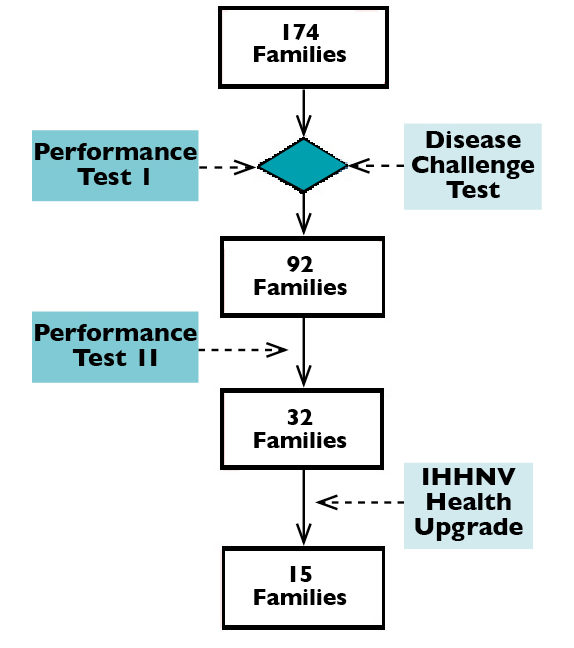
The study was conducted in an enclosed facility in Mexico where IHHNV infection was known to be present for over two years, using second-generation shrimp from a breeding program. For performance testing, 174 families were divided into three groups with 58 families per group and evaluated in raceways. Shrimp from each group were evenly distributed in four raceways, with about 40 shrimp/family/raceway.
The genetic selection scheme implemented is outlined in Fig. 1. Family selection was based on the results of two-phase performance testing with target harvest weights of 15 and 28 to 30 grams, respectively, as well as the results of disease challenge tests at the juvenile stage with Taura syndrome virus, white spot syndrome virus and infectious myonecrosis virus that were conducted in the Aquaculture Pathology Laboratory.
The IHHNV study was carried out for the 32 selected families after the second performance test phase. All selected survivors were individually weighed, eye tagged and pooled together by family.
About 30 shrimp per family were selected for IHHNV testing based on their sizes and visual health. Selected shrimp were held in flow-through raceways at a density of 15 per square meter to minimize the chance of further contamination.
Hemolymph was sampled from individual shrimp and submitted for dot blot hybridization testing on site. Shrimp found negative for IHHNV were then held separately in individual tanks. One week later, additional hemolymph samples were sent to a laboratory for polymerase chain reaction (PCR) tests.
Results
Of the total 1,029 shrimp screened for IHHNV by dot blot, 585 individuals showed negative. Of the 233 shrimp further tested by PCR, only 47 showed negative. An estimated 11.6 percent of the selected animals were negative by the first PCR.
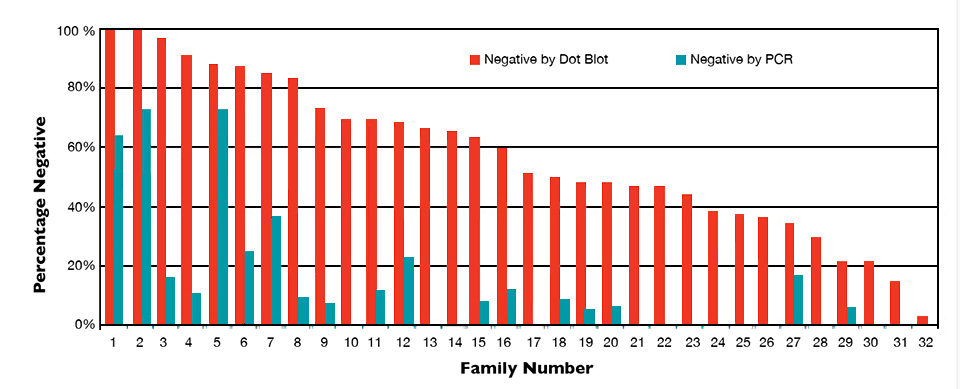
Statistical analysis showed no significant effect related to testing group, raceway and shrimp sex, but family had a significant effect on the IHHNV prevalence, as measured by percentages of the shrimp found negative by dot blot and PCR. Large variations of IHHNV prevalence were found among families (Fig. 2), with dot blots showing 3 to 100 percent and PCR levels ranging 0 to 100 percent. Although prevalence by PCR was only 11.6 percent overall, three families had more than 60 percent of their individuals negative to IHHNV, which suggested a fair chance to select IHHNV-free animals from the population.
Performance test results showed positive correlation between growth rates from the first and second phases, and slightly negative correlation between growth and survival rate. Although no correlation between the growth and tolerance of Taura syndrome virus was evident, there was strong positive correlation between growout survival and TSV tolerance.
The least square mean of harvest weight for shrimp found negative either by dot blot or PCR was significantly higher than that for positive shrimp and those not selected for testing (Fig. 3). Lack of IHHNV was positively correlated with harvest weight and negatively correlated with the coefficient of variation, but had no apparent effect on survival and TSV resistance.
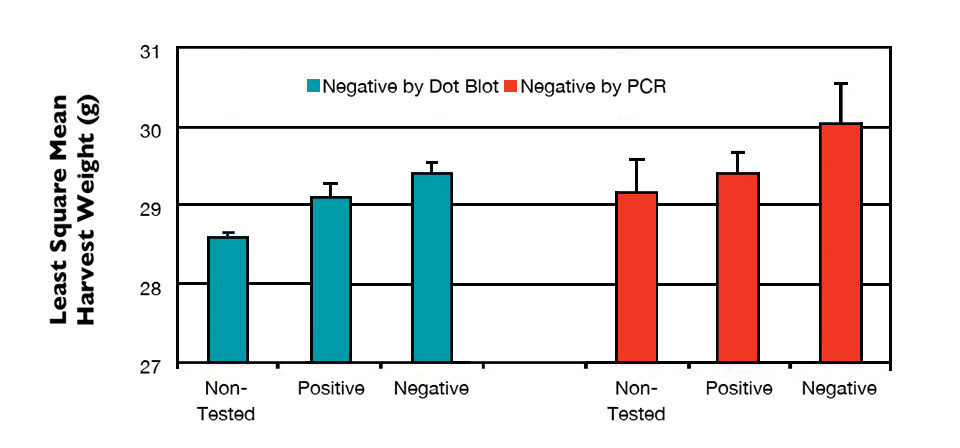
These results indicated that the severity of RDS and shrimp size variations were affected by IHHNV prevalence. At the individual level, shrimp without IHHNV infection or less viral load were larger than the severely infected shrimp. At family level, severe IHHNV infection resulted in lower growth and higher coefficient of variation.
It is worth noting that the IHHNV study was conducted using selected individuals from the best performance families, so the average 14 percent within-family coefficient of variation was smaller than the coefficient of 30 percent or more for a population with typical RDS. The PCR- percent (11.6 percent) didn’t reflect the whole population. Nevertheless, the high percentage of IHHNV-negative individuals in some families offered an opportunity to screen out IHHNV-free individuals from an infected population.
Perspectives
Practicality remains one of the major challenges to minimize potential contamination at each stage and to utilize clean animals, which typically are handled many times during screening for successful reproduction. The process requires systematic planning to incorporate many important aspects, as well as detailed step-by-step implementation.
IHHNV tolerance and resistance are two distinct genetic traits. Large between-family variation in IHHNV susceptibility, as shown in this study, may provide a genetic basis to increase IHHNV tolerance and to explore the potential of developing a line refractory to IHHNV infection via genetic selection.
(Editor’s Note: This article was originally published in the November/December 2009 print edition of the Global Aquaculture Advocate.)
Authors
-
Hui Gong, Ph.D.
College of Natural and Applied Sciences
University of Guam
Mangilao, Guam 96923 USA[117,100,101,46,103,111,117,46,109,97,117,103,117,64,103,110,111,103,104]
-
Donghuo Jiang, Ph.D.
College of Natural and Applied Sciences
University of Guam
Mangilao, Guam 96923 USA -
Adrian Rojas
San Juan de los Lagos
Jalisco, Mexico
Tagged With
Related Posts

Health & Welfare
Measuring susceptibility differences to IHHNV infection
This study evaluated the susceptibility to IHHNV in three different shrimp batches by measuring infectivity titers. Each batch showed a different infectivity titer, therefore, each batch had a specific susceptibility to IHHNV.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Innovation & Investment
Aquaculture America 2017: Communication key to the future
This year’s Aquaculture America in San Antonio, Texas, provided significant learning and networking opportunities. It successfully brought together 14 U.S. aquaculture organizations and more than 1,600 participants from Europe, Asia, Africa and Australia.

Aquafeeds
Biosecurity protocols needed for shrimp feeds, feeding practices
Shrimp aquafeeds – live, fresh or formulated – should not be an entry point of potential pathogens to the shrimp and/or to their culture systems.


