Time-consuming and labor-intensive method appropriate for small-scale lab or field studies

The ability to monitor the performance of individual shrimp or groups of shrimp in a population provides researchers with a valuable tool in physiology and behavior studies. It is also invaluable in genetic improvement programs to increase selection intensity and eliminate environmental effects, because all individuals are evaluated in a common environment.
External, internal tags
A variety of external and internal tags have been developed over the past several decades to enable researchers to monitor aquatic animals. External tags can be effective for individual identification of some fish species, but can present problems in crustaceans because they can interfere with the molting process or be lost during ecdysis. Internal tags typically are less intrusive and not lost during ecdysis, but visual identification can be more difficult.
Tagging crustaceans
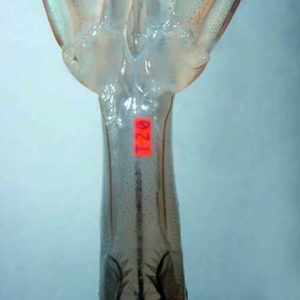
Internal tags used with decapod crustaceans include coded wire tags in juvenile lobsters and juvenile mud crabs; visible implant elastomer tags in juvenile white shrimp, L. vannamei (Fig. 1); and microchip passive integrated transponder tags in postjuvenile prawns.
Coded wire tags can be used for individual identification, but the animals they are implanted in typically have to be sacrificed to recover the tags. Elastomer tags typically are used for batch coding to monitor family or group performance, but individual identification is limited with these tags. Passive integrated transponder tags are relatively expensive, require an external reading device, and pose problems associated with biocompatibility in smaller specimens.
Polyester visible implant tags
The development of a polyester visible implant tag by Northwest Marine Technology, Inc. of Shaw Island, Washington, USA provides a tagging system with individual alphanumeric descriptors that can be applied to small aquatic animals. These tags are implanted internally into transparent tissue and are externally readable.
Polyester visible implant (VI) tags have been used successfully in fishery applications involving field studies with sea-run cutthroat trout and juvenile brown trout, and in cage studies with juvenile Atlantic salmon. A new version of the tag, the “soft VI-alpha,” recently was developed using a pliable medical-grade elastomer pigmented with nontoxic fluorescent material. Various colors and codes allow over 40,000 individual marks to be identified. This technology has been used in freshwater and red swamp crayfish.
Tag retention and visibility
Recently, researchers at the Oceanic Institute in Hawaii, USA evaluated the efficacy of the soft VI-alpha tagging system in juvenile and subadult L. vannamei under both laboratory and field conditions. The objective was to evaluate tag retention and visibility of two tag sizes (standard format, 1.0 x 2.5 mm, and large format, 1.5 x 3.5 mm).
For both juvenile (mean weight 2.7 g) and subadult (mean weight 21.5 grams) shrimp, tags were inserted through the ventral surface of the abdomen (Fig. 2). Shrimp were maintained in lab tanks or outdoor ponds for evaluation.
For juvenile shrimp in the lab, tag retention was 95 percent for both tag sizes with 82 percent readability after shrimp reached a mean weight gain of 10.5 grams over 42 days (Table 1). Under field conditions, there was a 70.5 percent recovery of tagged juveniles with 81 percent readability after shrimp exhibited a mean weight gain of 21.1 grams over 140 days (Table 2).
For subadult shrimp in the lab, overall tag retention was 99.2 percent for both tag sizes with 95 percent readability weight gain of 4.7 grams over 75 days (Table 3). Under field conditions, there was an 84 percent recovery of tagged subadults with 84 percent readability after an average weight gain of 20 grams over 110 days (Table 4).
Arce, Tag and animal performance for juvenile shrimp, Table 1
| Tag Size | Initial Weight | Final Weight | Weight Gain | Tag Retention | Tag Readability | Survival |
|---|---|---|---|---|---|---|
| Standard | 1.6 g | 13.0 g | 11.4 g | 97.8% | 76.5% | 90.0% |
| Large | 1.6 g | 11.3 g | 9.7 g | 92.3% | 87.5% | 80.9% |
Arce, Tag and animal performance for juvenile shrimp, Table 2
| Tag Size | Initial Weight | Final Weight | Weight Gain | Tag Recovery | Tag Readability |
|---|---|---|---|---|---|
| Standard | 3.7 g | 24.9 g | 21.2 g | 74% | 82% |
| Large | 3.9 g | 24.9 g | 21.0 g | 67% | 80% |
Arce, Tag and animal performance for subadult shrimp, Table 3
| Tag Size | Initial Weight | Final Weight | Weight Gain | Tag Retention | Tag Readability | Survival |
|---|---|---|---|---|---|---|
| Standard | 21.4 g | 26.6 g | 5.2 g | 100.0% | 97.0% | 98.3% |
| Large | 21.3 g | 25.4 g | 4.1 g | 98.4% | 93.0% | 95.0% |
Arce, Tag and animal performance for subadult shrimp, Table 4
| Tag Size | Initial Weight | Final Weight | Weight Gain | Tag Recovery | Tag Readability |
|---|---|---|---|---|---|
| Standard | 21.9 g | 41.6 g | 19.6 g | 83.3% | 80.0% |
| Large | 21.5 g | 42.5 g | 20.5 g | 85.0% | 88.2% |
Conclusion
The soft VI-alpha tagging system can serve as an effective research tool, but is appropriate for small-scale lab or field studies only, because tag insertion is time-consuming and labor-intensive.
For research and genetic programs that monitor many individuals of known pedigree, the use of genetic tags or markers such as microsatellites (Fig. 3) and single nucleotide polymorphisms is likely the most effective approach. In addition, genetic markers can assess the genetic diversity of captive populations to avoid inbreeding and establish markers linked to commercially important traits.
(Editor’s Note: This article was originally published in the December 2002 print edition of the Global Aquaculture Advocate.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Steve M. Arce
The Oceanic Institute
41-202 Kalanianaole Hwy.
Waimanalo, Hawaii 96795 USA[103,114,111,46,101,116,117,116,105,116,115,110,105,99,105,110,97,101,99,111,64,101,99,114,97,115]
-
Shaun M. Moss, Ph.D.
The Oceanic Institute
41-202 Kalanianaole Hwy.
Waimanalo, Hawaii 96795 USA
Related Posts

Health & Welfare
Atlantic cod genomics and broodstock development project
The Atlantic Cod Genomics and Broodstock Development Project has expanded the gene-related resources for the species in Canada.

Health & Welfare
Catfish genetics and breeding
Although research on catfish genetics began in the 1960s, applications of genetic improvement in aquaculture have lagged behind other animal industries.

Health & Welfare
Diversity loss in captive-breeding programs for shrimp
It is important for any breeding program that founding populations be large enough to incorporate the diversity of the wild animals.

Health & Welfare
Genetic response to selection in channel catfish
USDA and partners are developing channel catfish that exhibit superior growth, fillet yield and resistance to enteric septicemia. Although ongoing research continues to select the USDA lines for better performance, studies comparing the strains have been inconclusive.


