Current knowledge of microalgae-derived oils with nutritional value and the strategies to improve their yields
The commercial production of microalgae has received great attention for different purposes during the last decades. These microorganisms have been largely investigated as a source of biofuels, bioactive components for cosmetic and cosmeceutical formulations, and high-value pharmaceuticals. Microalgae appear also suitable as fertilizers because of their water-binding capacity and their ability to release macro- and micronutrients to the surrounding soils at low rates. They are currently being investigated for aquatic environments’ bioremediation, known as phycoremediation.
Microalgae have also been studied because of their potential for the production of healthy food and feed supplements. Microalgal biomass can be used as raw material for third-generation biorefinery processes, which foresee its conversion into a wide spectrum of marketable products and energies, minimizing costs and waste production. To date, various companies have adopted this approach and are able to grow microalgae at pilot scale and to produce different kinds of commodities (mainly energy carriers, pigments and polyunsaturated fatty acids, or PUFAs) from the same biomass, for various biotechnological sectors.
This article – adapted and summarized from the original publication (Santin, A. et al. 2021. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26(24), 7697) –describes the current knowledge of microalgae-derived oils with nutritional value, the strategies to improve their yields, and the main bottlenecks related to large-scale production by the food and feed industries.
Polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) are fatty acids (FAs) classified into two main groups on the basis of the length of their carbon backbone: short-chain polyunsaturated fatty acids (SC-PUFAs), with 16 or 18 carbon atoms, and long-chain polyunsaturated fatty acids (LC-PUFAs) with more than 18 carbons.
Consumption of PUFAs is related to various health benefits for humans, including the improvement of metabolic rates, the regulation of blood pressure and glucose levels, and protection against numerous diseases, including some types of cancer. Clinical and epidemiological studies have shown that an eicosapentaenoic acid (EPA)-rich diet contributes to minimize risks of cardiovascular diseases. Docosahexaenoic acid (DHA) and arachidonic acid (AA) are important to avoid impairments in infant brain development and cognitive deficiency. EPA and DHA may also lower the risks of obesity in both humans and animals] and can prevent chronic inflammatory diseases.
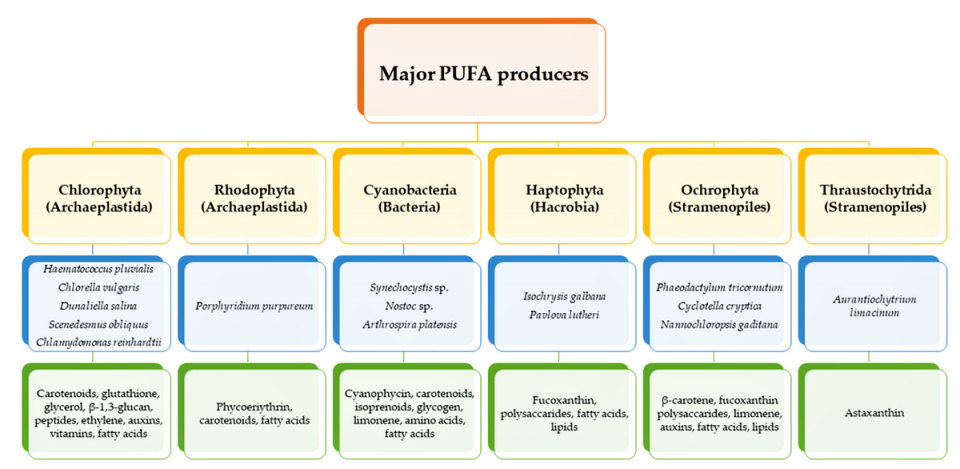
Humans, as other mammals, are unable or poorly able to synthesize some essential PUFAs, such as linoleic acid, and α-linolenic acid, which are precursors of AA and DHA, respectively. Therefore, a direct uptake of these compounds from external sources is necessary. These sources include aquatic organisms (fish, molluscs and crustaceans), animal-derived commodities (meat, milk, eggs), fungi, bacteria and vegetable sources, such as some plants and microalgae. The main microbial sources, which are able to produce highly valuable lipids for nutraceutical purposes, are summarized in Fig. 1.
Microalgae-based technology
Microalgae-based technology has several advantages that overcome numerous constraints related to the cultivation of other organisms and/or natural sources. They do not require arable land, thus they do not compete with agricultural crops for space, and they have growth rates higher than those observed in terrestrial plants.
Additionally, microalgal growth is an environmentally safe process, not requiring herbicides and pesticides for maintenance. Their growth can be directly related to carbon dioxide fixation and can also contribute to the reduction of other greenhouse gases in the atmosphere such as nitrogen and sulfur oxides. Microalgal biomass does not require cost- and time-consuming pre-treatments for the extraction of biomolecules and lends itself to different extraction techniques, some of which minimize or avoid the use of solvents.
Some microalgae-derived products are currently available on the market. Algal biochemical composition and the yields of their high-value lipids can be tuned through regulation of growth conditions and/or genetic transformations aimed at modifying algal metabolism. Short-chain fatty acids with lower pureness and low unsaturation degrees, which are inadequate for the production of food or feed commodities and for pharmaceutical purposes, are still useful for biodiesel production. However, the high cost of microalgal biofuel compared to that of fossil sources still prevents its production. Conversely, different industrial plants producing food or feed supplements are growing worldwide, even if there are still few species that have found a market.
Genetic engineering
In recent years, genetic and metabolic engineering have been used to improve different features of organisms, for example, increasing the production of high-added-value biomolecules. High-throughput sequencing technologies and genetic transformation techniques greatly contributed to enhance lipid production, and, in particular, PUFAs’ accumulation, not only in microalgae but also in plants, yeasts and bacteria.
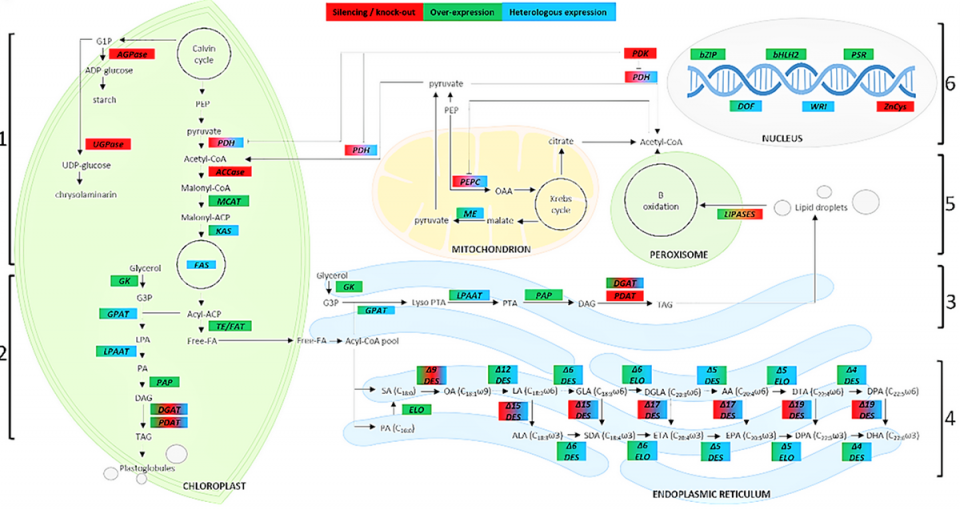
A number of strategies can be employed to regulate genes involved in lipid metabolism and PUFAs’ biosynthesis, generally perturbing gene expression by overexpressing or silencing endogenous genes; common strategies are the overexpression of enzymes in the biosynthesis pathway, the perturbation of the regulation of related biosynthetic pathways, the block of competing pathways, and, in some cases, the use of a multi-gene transgenic approach.
Several researchers have emphasized the possibility of heterologous [derived from a different organism] protein expression in different organisms: This means expressing microalgae proteins in other microalgae or in other organisms or expressing proteins from other organisms in microalgae. These studies have allowed characterizing a large number of genes involved in different metabolic pathways and identifying interesting targets for increasing PUFAs’ production. Regarding the enhancement of the fatty acid biosynthetic pathway, the main approach includes the direct modification of fatty acids’ composition through the manipulation of the genes involved in fatty acid biosynthesis (Fig. 2), generally by increasing the expression of one or more enzymes.
Microalgae-derived lipids
In this review article we highlight the potential of microalgae-derived lipids as sustainable sources or food and feed. The modulation of nutrient supply (typology and/or concentrations in culture media) and of physical parameters (temperature, light, irradiance) can enhance the yields of specific lipids; culturing conditions that maximize production and/or productivity of highly valuable lipids can be species-specific, and, in some cases, specific growth strategies (such as a two-stage cultivation system) are required to maximize both biomass and lipid content.

Currently, large-scale production of microalgae-derived lipidic commodities for food and feed industries is mostly based on cultivation in open ponds, since they represent the most economical alternative for massive growth. On the other hand, closed photobioreactors should be intended for final products requiring high quality levels and very low or zero concentrations of contaminants, such as nutraceuticals and pharmaceuticals, but their rigid condition control is still very expensive. An interesting strategy is to first extract their lipids for the production of biodiesel, due to their high lipid contents, and then process the remainder lipid-free material as protein- and fatty acid-rich products. Finally, we believe that a further effort to decrease operational costs through sustainable processes and nutrient recycle is required by phycological research.
An additional advantage of indoor photobioreactors compared to outdoor open ponds is the strict regulations on genetically modified organisms, including microalgae. Genetic engineering of microalgae contributed to improve specific biochemical features of some species and culturing the resulting strains rather than the corresponding wild types proved to be more economically viable. Since the accidental introduction of genetically modified microalgae in the environments can lead to dramatic consequences, comparable to those caused by some imported species, legislation does not allow outdoor cultivation of such organisms in most countries. In contrast, genetically modified microalgae could be safely cultured in indoor systems, guaranteeing an aseptic separation between the inner and the outer environment is provided.
Perspectives
Microalgal production of PUFAs has much potential and opportunities for further development, but at the same time needs to address some key challenges, including needed technical breakthroughs, access to venture capital, and regulatory, academic and industrial training (with cooperation between all), and reduction of biomass production cost.
Although commercial-scale cultivation of microalgae is quickly improving, production costs of microalgal biomass is yet far from competitive. The main reasons behind the large production costs are related to the small relative content of PUFAs within microalgal biomass, which may depend on the cell growth and on the extraction methods.
Microalgae are the most sustainable and environmentally safe source to be exploited for the production of PUFAs for human consumption, as well as for feeding terrestrial and aquatic animals. However, the world has not yet fully exploited this potential, as other sources of PUFAs are still more economically advantageous, with already optimized production and extraction methods and high yields. Therefore, research in microalgal biotechnologies should focus on searching for novel strategies to minimize production costs and to increase the lipid yields, in the coming decade. Different aspects can be addressed to make microalgal production viable at an industrial scale, like the optimization of growth conditions, upstream processing to increase PUFAs productivities and selecting between different downstream processing approaches to decrease production costs.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Anna Santin, M.S.
Stazione Zoologica Anton Dohrn, Naples, Italy
-
Monia Teresa Russo, Ph.D.
Stazione Zoologica Anton Dohrn, Naples, Italy
-
Maria Immacolata Ferrante, Ph.D.
Stazione Zoologica Anton Dohrn, Naples, Italy
-
Sergio Balzano, Ph.D.
Stazione Zoologica Anton Dohrn, Naples, Italy; and Department of Marine Microbiology and Biogeochemistry, Netherland Institute for Sea Research, The Netherlands
-
Ida Orefice, Ph.D.
Stazione Zoologica Anton Dohrn, Villa Comunale, 80121 Naples, Italy
-
Angela Sardo, Ph.D.
Corresponding author
Stazione Zoologica Anton Dohrn, Naples, Italy; and
Istituto di Scienze Applicate e Sistemi Intelligenti “Eduardo Caianiello,” Pozzuoli, Italy[116,105,46,110,122,115,64,111,100,114,97,115,46,97,108,101,103,110,97]
Tagged With
Related Posts

Health & Welfare
Algae shows promise as alternative DHA source in rainbow trout diets
A growth trial in Canada evaluated the use of algae biomass to increase the concentration of long-chain polyunsaturated fatty acids in the tissues of rainbow trout.

Intelligence
Omega-3 fatty acid composition of Atlantic salmon fillets
Study searched for genetic variants associated with omega-3 fatty acid content in Atlantic salmon, to identify genes underlying genetic variation.

Aquafeeds
Improving the lipid profile of black soldier fly larvae
Black soldier fly larvae fed with marine-based substrates displayed an improved lipid profile with higher polyunsaturated fatty acid levels.

Health & Welfare
Long-chain PUFA requirements of juvenile California yellowtail
This study shows that soybean oil can totally replace fish oil in California yellowtail aquafeeds without affecting production performance and reducing the omega-3 fatty acid profile of fish tissues, as long as acceptable levels of ARA and DHA are provided.



