Aker BioMarine clears regulatory hurdle after 10 years

Norway-based Aker BioMarine has announced that the U.S. Food and Drug Administration (FDA) has approved its krill-based protein meal products for use in freshwater and marine farmed salmonid feeds.
Aker’s QRILL™ Aqua and QRILL™ High Protein meal contains astaxanthin, a natural source of the pigment that enhances salmon flesh color, and the FDA classifies it as a color additive.
“We have been waiting for this approval for a long time and are very excited to see this come to life,” said Sigve Nordrum, executive VP of animal health and nutrition at Aker BioMarine. “We serve the aquaculture industry across the globe and now we are excited to do the same in North America. For years, feed companies and fish farmers in this region were well aware of the benefits of krill for fish feeds and salmon, therefore they have been demanding these products for their North American operations. We look forward to working with our customers in this market to support the production of high-quality, sustainable and healthy fish.”
Made from Antarctic krill, QRILL Aqua functions as a feeding stimulant leading to increased feed uptake and enhanced growth. Aker says that QRILL Aqua is also proven to improve health, stress tolerance and fillet quality. QRILL High Protein is a protein-rich product used in formulated diets for farmed fish and shrimp, leading to increased feed uptake and enhanced growth.
Will krill fulfill its promise as an aquaculture feed ingredient?
According to Mariana Naum, Ph.D., team lead-strategic communications for the Center for Food Safety and Applied Nutrition at the FDA, the agency amended the color additive regulations to provide for the safe use of Antarctic krill meal on May 10. Antarctic krill meal is composed of the ground and dried tissue of Euphausia superba, with or without the lipid fraction, intended for use in the feed of salmonid fish, to enhance the color of their flesh. “We took this action in response to a color additive petition (CAP) submitted by Aker BioMarine Antarctic AS (Aker BioMarine or petitioner). The final rule became effective on June 10, 2022,” she told the Advocate.
Follow the Advocate on Twitter @GSA_Advocate
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Responsible Seafood Advocate
[103,114,111,46,100,111,111,102,97,101,115,108,97,98,111,108,103,64,114,111,116,105,100,101]
Related Posts

Aquafeeds
Alternative ingredients for tilapia aquafeeds
The increasing production of tilapia is increasing the demand for fishmeal as a protein source for formulated aquafeeds.

Aquafeeds
Krill meal performs well in shrimp feed experiments
A study of experimental diets for juvenile shrimp showed a halving of fishmeal usage. Limited inclusion of krill meal offset other expensive ingredients.

Health & Welfare
Krill meal use reduces other costly ingredients in shrimp study diets
Due to its high protein and highly unsaturated fatty acids content, krill meal can be an effective ingredient in aquafeed.
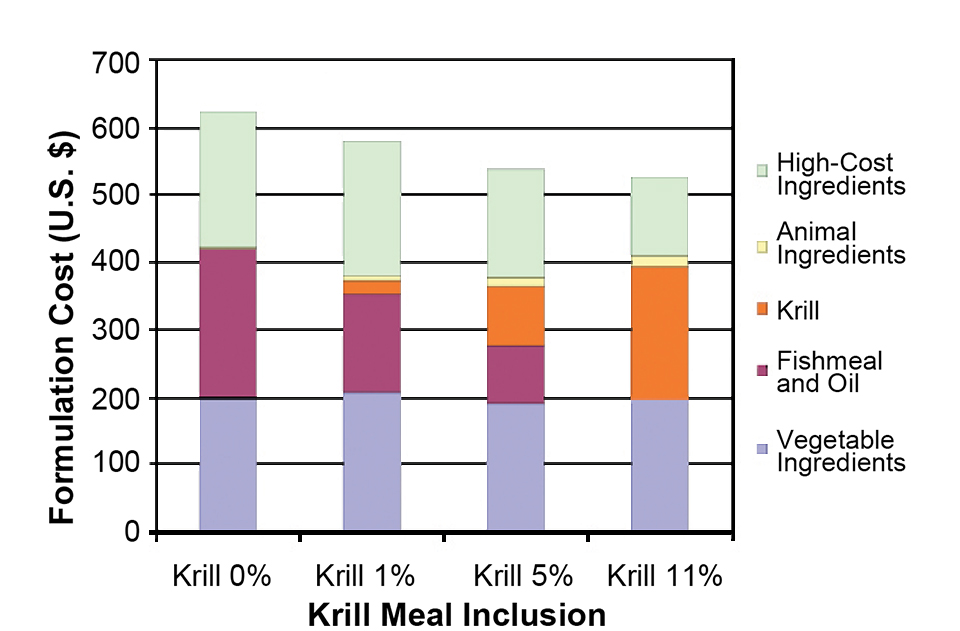
Aquafeeds
Study finds krill meal cost-effective ingredient in shrimp feed
In a study, krill meal incorporated into shrimp diets replaced fishmeal and soy lecithin with no significant effect on shrimp performance.



