Shrimp farming expert Robins McIntosh expounds on Enterocytozoon hepatopenaei (EHP) and its relevance to the global shrimp farming industry

Robins McIntosh, executive VP at Thailand-based Charoen Pokphand Foods Public Company and a longtime prominent figure in shrimp farming globally, spoke to the Advocate recently regarding the nuanced history of Enterocytozoon hepatopenaei (EHP), one of the primary shrimp diseases in Asia and globally.
Originating in black tiger shrimp (Penaeus monodon) in the late 1980s, EHP gained definitive recognition in Pacific white shrimp (Litopenaeus vannamei) in 2004 in Thailand, but it soon extended globally, spreading with an elusive nature, often escaping identification due to diagnostic limitations.
McIntosh discusses the impact of EHP on shrimp hatcheries while emphasizing the importance of biosecurity measures, certified broodstock and the intricacies of disinfection and surveillance in efforts to mitigate its impact.
He also unveils the symbiotic relationship between EHP and White Feces Disease, spotlighting the role of anaerobic bacteria, particularly Propionigenium. Stress emerges as a catalyst, with dissolved oxygen levels crucial in managing outbreaks. McIntosh also addresses the presence of EHP in the Americas, attributing disease severity differences to stress and stocking densities.
The following is an edited transcript of our conversation with Robins McIntosh.
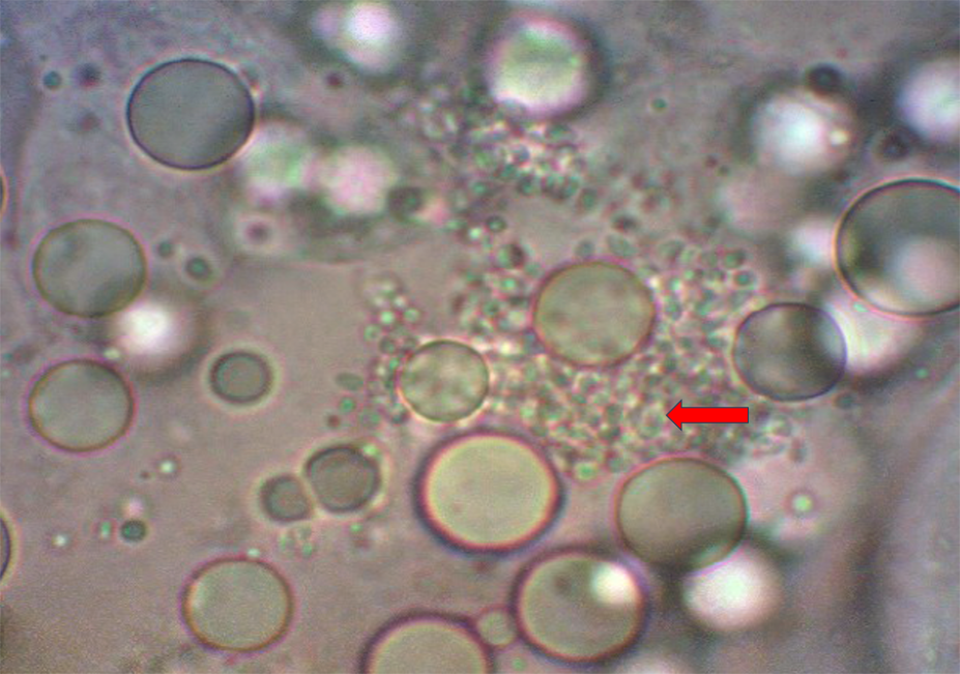
The history of EHP
First, a history of EHP. Many people call it an emergent disease. It’s an emergent, serious disease, but it’s been around for a while. There are records as early as the late 1980s in black tiger shrimp (Penaeus monodon) that look like they were probably EHP. But it was definitively described in monodon in Thailand in 2004. And my first run-in with EHP was in Thailand was 2011; there were definite cases where EHP was causing disease.
Then, in 2012 during the GOAL meeting in Bangkok, I took Dr. Donald Lightner to one of our labs and I showed him his first EHP in diseased shrimp that were from Thailand. We observed clusters of small elements in the HP. He had never seen it. He really couldn’t identify it. We just knew that these things were appearing in the hepatopancreas (HP) of affected shrimp and other issues were also happening at the same time. So, at that point, it was there, but it was kind of an undiagnosed issue. It spread quickly into China or was in China concurrently in 2012–2013, where the shrimp farming issue was also having major, undescribed issues – unidentified at the time, but in hindsight, all of this was EHP.
Donald Lightner, influential figure in shrimp aquaculture, remembered
At the same time, we were finding structures in the intestine that resembled gregarines (a common group of parasitic protozoa infecting shrimp and other aquatic animals).
In time we began to understand that these gregarine-like structures, these ATMs (Aggregated Transformed Microvilli), are associated with EHP and were observed in many places and often went undiagnosed. There was no PCR at this time – it was all done through microscope examination looking for these little spores, what we now know are EHP spores in the HP. Again, very difficult to identify, but possible once you got a good eye for them.
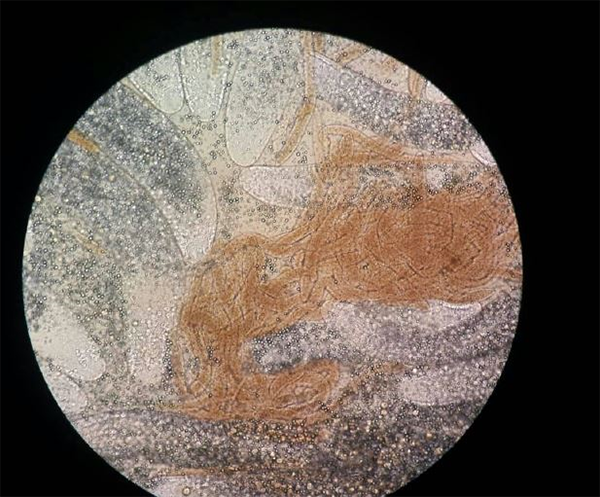
It was also noted that these shrimp were part of slow-growing populations with higher coefficients of variation (CVs) but did not seem to result in any survival issues.
Now, using spores as a diagnostic is not always 100 percent because there are two phases to EHP. There is the sporulated phase where you have the spores and then there is the sporophytes, which look like HP tissue, so you can’t identify them. For the sporophytes, you have to use PCR or in situ hybridization to identify them. If it’s in the sporophyte phase, that oftentimes has led to [misidentification] of EHP.
As we go forward in time, we start seeing EHP appearing in other locations, certainly in Vietnam, in the Philippines, Indonesia … it really started hitting India in 2015 or 2016, and always with the movement of broodstock. The transmission of EHP is through shrimp, the active passage that we know of now. There may be other active carriers, but these are under-identified or under-diagnosed at this time. Certainly, most of the passage around Asia and the world has probably been through the movement of shrimp. And since PCR was not readily available for EHP, through broodstock movement it found its way into many, many countries because it was undiagnosed.
Dr. Lightner was relying strictly on microscopy at that point, and there were misses. In the diagnosis, it wasn’t until the implementation of the PCR primers for EHP in 2014 that we were able to diagnose EHP with certainty. Before then, the first primers were generic for enterocytozoa and produced many false positives. The specific EHP primers were developed by Dr. Tim Flegel’s lab in concert with other researchers in England. These primers were based on detecting the spore wall of this particular EHP, so it was much more specific and with few false positives. Before these specific primers, there were many times you would test shrimp feeds, feed ingredients like krill meal and fishmeal, different other substances and results would be falsely positive for EHP.
So, what happened in my case? For our broodstock we had a nucleus breeding program, a very strict one for biosecurity: Any positive results for EHP and those animals were out. Now, because EHP is in the HP, the non-destructive way of testing has been through feces samples, so you’re only going to be testing for spores. So, if there are no spores, you can’t get a positive, which could lead to a false negative. But on the other hand, if you’re feeding the broodstock a feed that is positive for EHP and you’re testing the feces, the feces are positive and that’s a false negative. Before we really understood this – and this is probably around 2014 – one time we got a report that one of our broodstock raceways was positive for HP. I had them tested again and it came back positive again. And so, we had to sacrifice the shrimp in several broodstock raceways, valued at probably over U.S. $1 million.
But then I had the idea of testing the HP next time we had a similar situation, and well, the HP was negative. Then the feed was tested and the feed was positive, we realized that you could make bad mistakes by just testing feces. Because if the feed is positive, the feces will be positive and today there are still feeds that are positive, and so you have to recognize that in testing. Also testing positive are krill meals and some fishmeals.
If you get a positive, you really must go in and test HP tissue to determine if EHP is present or whether it’s just in the intestine passing through from something the shrimp has eaten that is not really EHP. Now the more specific primer does reduce that probability. But it’s always a probability that just because it’s in the feces, it could be under-diagnosed because the shrimp is not forming spores, or it can be over-diagnosed because what you’re feeding the shrimp is also positive for EHP. For true verification you should always test the hepatopancreas, and an homogenate of the entire HP, as the infection may be localized in the HP and not equally distributed throughout the HP.
And because spores are not equally distributed and because they may be few in number, the histological exam must be done with extreme patience observing many slices through the HP to make a diagnosis of not present. And in the beginning before PCR, many infections were missed because by using normal staining techniques the spores were difficult to see – but the slides were aged a few weeks and the spores became much more visible.
Historically, EHP by itself causes slow growth and high coefficients of variation (CVs) in size of the shrimp.
Originally the disease caused slow growth with a high size variation (or CVs over 25 percent). Shrimp from CP genetics would minimally average a growth rate of over 0.18 grams per day. Anything under 0.15 average daily gain (ADG) would be in most all cases be a result of an EHP infection. The defining characteristic of EHP was a size variation similar to what you saw with Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV) and Runt Deformity Syndrome (RDS).
Current status of IHHNV infection in Peru’s and Ecuador’s shrimp industries
Another thing that I began to notice was that EHP would either hit early or it would hit later in the shrimp production cycle. If a farm was reporting slow growth at 30 days, maybe at 30 days their normal size was 2.5 grams, but now at 30 days, the shrimp size would be less than one gram – this generally was a good indication that the infection came from the hatchery. If the EHP came from the environment, growth was not impacted until 45 to 50 days. And if it was from the environment, many farms in Vietnam and other places would develop the strategy of trying to outrun the disease, to produce a harvestable size shrimp in 70-80 days.
Later, using real-time PCR, we learned that the level of EHP DNA determines if there is disease or no disease. At concentrations of 101 to 103, the shrimp is merely a carrier of EHP with no discernible disease (slow growth), but as the levels increase to over 103 shrimp begin to grow slower. If the levels reach 106 to 108, shrimp growth is significantly reduced.
For EHP that originates from the hatchery and slows growth before 30 days, there’s no way of getting an economic return. For the first 30 days, shrimp growth is a good clue as to whether the EHP is originating in the hatchery or is coming from the pond environment.
It must also be assumed that if EHP is found in either a hatchery or pond, the infection will remain in the hatchery or in the pond unless specific measures are taken to remove the infective spores from the hatchery and or the pond.
Shrimp hatcheries and EHP
If a hatchery is suspected to have EHP or has EHP, it must be disinfected, and the spores are difficult to neutralize. There are reports that 35 to 40 ppm of chlorine, potassium permanganate or formalin will all disinfect in laboratory conditions; but experience is that these disinfectants are not effective in the real world, a world with protective biofilms, etc.
In hatcheries, to disinfect a surface you must use either an acidified chlorine (200 pm/pH 4.0) solution or a base such as sodium hydroxide solution (pH >12. These solutions will break down biofilms and dissolve the spore walls. To really clean a hatchery, the entire facility must be disinfected and that includes reservoirs and pipes.
If a hatchery has EHP, the operator should determine how the EHP got into the hatchery. The most obvious ways are 1) broodstock, 2) live or fresh broodstock feeds (anything that is a filter feeder can bring in EHP), and 3) source water. To test the water, a couple of liters of water can be filtered through a Whatman filter, which can then be tested by PCR. Pond-reared broodstock from infected regions should never be used and it is highly preferable to use genuine certified SPF broodstock from a recognized source. Another major source of infection are filter feeders such as polychaetes, bivalves and adult artemia that are often used as broodstock feeds in maturation. It is best to either not use these or use cultured material from known clean sources.
In Thailand, we would monitor the water once a month or once every week, depending on whether you know the history of the hatchery or not. And you must have certified SPF broodstock. It’s really not just about a PCR test. It is about the history of the facility it came from. So, if the broodstock is coming from a facility with an EHP-free history, it is not going to have EHP. And the broodstock feces must also be tested, as there’s a chance that you will miss it initially because it may be a sporophyte. Also, the levels of the spores or the DNA are important to get positive tests, and again with broodstock you can’t test the hepatopancreas and keep the animal alive. So, it’s really important to get the broodstock from a truly certified facility with a long history.
Once we have clean broodstock, we bring it into a clean hatchery. The water is clean. We’re not in an EHP area, so the water is clean. Now we just have to make sure that we do not bring in a broodstock feed that has EHP. We do not want live polychaetes, live artemia or artemia biomass that has been produced in an EHP zone. And it certainly is never a bad thing to test all of these by PCR to ensure that the feeds are clean.
Your best bet is cultured SPF or certified pathogen-free polychaetes. Or potentially you can cook the polychaetes 75 to 80 degrees-C. In my opinion, this deteriorates the polychaetes, but it will deactivate the EHP spores if you have to use polychaetes. Squid is generally safe, but I would not be using any bivalves (oysters, clams, mussels), as anything that filter-feeds can filter and retain EHP spores and be a carrier of EHP. Polychaetes feed in the sediments, which can bring EHP spores into their intestines. Those are all ways of infecting your broodstock with EHP.

If you’re careful with the feed, you’ve disinfected the hatchery, you’ve got a clean water source, then you should be able to remain EHP-free in the hatchery. Now, if you are in a highly infected area like some hatchery areas of India, or Vietnam or Thailand where there is a mingling of hatcheries and farms and you got EHP in the environment, you’re probably going to find EHP in the hatchery water. So that water must be disinfected.
This can be very difficult because of the biofilms and other factors. Yes, we say we can do it, but historically or observationally, when I look at the histories of hatcheries, they generally do not disinfect properly. If it was as easy as applying 40-ppm chlorine all over, we wouldn’t have the big problem we have at hatcheries today. But I think it’s more difficult than just using 40-ppm active chlorine. So really the only way to truly reduce the probability of EHP infecting from the water in a hatchery is through physical filtration, not chemical disinfection, for spores. These spores are less than two microns in size. You must use an ultra-filtration unit that can remove 2-micron particles from the water. And once installed the filter must be maintained to remain effective.
If you can properly filter the water, and have clean broodstock animals, clean feed, the water is either clean or we’re putting it through some mechanism to ensure that no spores enter the facility, then the hatchery should produce EHP-free postlarvae (PLs). But hatcheries must also always have a surveillance program. The surveillance should be either routine or certainly by periodic checking of the PLs.
Now, another problem when you’re checking PLs – unless the hatchery is just really, really infected and that does happen – is if a hatchery has just a low-level infection of EHP, so it’s going to be probably below detection level. So, what I have always done was stress the PLs, so now I am going to discuss how stress brings out EHP and magnifies the effect. So, if you take a bag of PLs and you ship them to yourself for a trip time of 24 to 28 hours, the stress of that shipping will bring out the EHP. It will replicate the EHP to the point that it’s easy to detect with PCR. In monitoring hatcheries we would ship PLs to ourselves and then test the animals. If the test is negative, we would have greater confidence that the animals are clean and are not going to carry EHP.
So, about 11 or 12 years ago, we started observing under the microscope what we call Aggregated Transformed Microvilli (ATM), the sloughing intestine. Later we discovered these ATMs were associated with EHP infections that were releasing massive numbers of spores and sloughing HP tissues.

White feces became an issue in 2014 and has continued to become more of an issue with time. But 2014 was not the first time that white feces were reported with disease. The first time was in the early part of the 2000s in P. monodon culture. White feces are always with an EHP infection. And when white feces appears, the disease is more serious than EHP alone, and now survival also becomes negatively impacted. And the question becomes what causes the white feces. We know EHP is required, but what else?
I noticed that white feces most often happened with bad bottoms and with ponds with broken plastic liners. Broken liners develop anaerobic conditions beneath the liners, which spill into the ponds. And this often happened with the very thin liners farms used to save costs. In these ponds you were most likely to find white feces with EHP. And if farmers treated such ponds with photosynthetic bacteria, the white feces could be mitigated or even reversed.
So, to look for the cause of white feces, I started concentrating on anaerobic bacteria. And so, our lab went out and collected anaerobic bacteria; we procured anaerobic jars so we could culture anaerobic bacteria in the lab, and we would find white feces. We would culture the bacteria from the white feces in our anaerobic jars, isolate those bacteria and then add those bacteria to shrimp with and without EHP. When we added certain bacteria to shrimp together with the EHP, we got white feces very fast. If we added the bacteria without EHP, there was never white feces, so there clearly was a connection. When we identified the bacteria that created white feces with EHP, it was an anaerobic bacteria called Propionigenium. Since our work with Propionigenium, other bacteria in the genus Vibrio have also been reported to produce white feces, but also only in the presence of EHP infections.
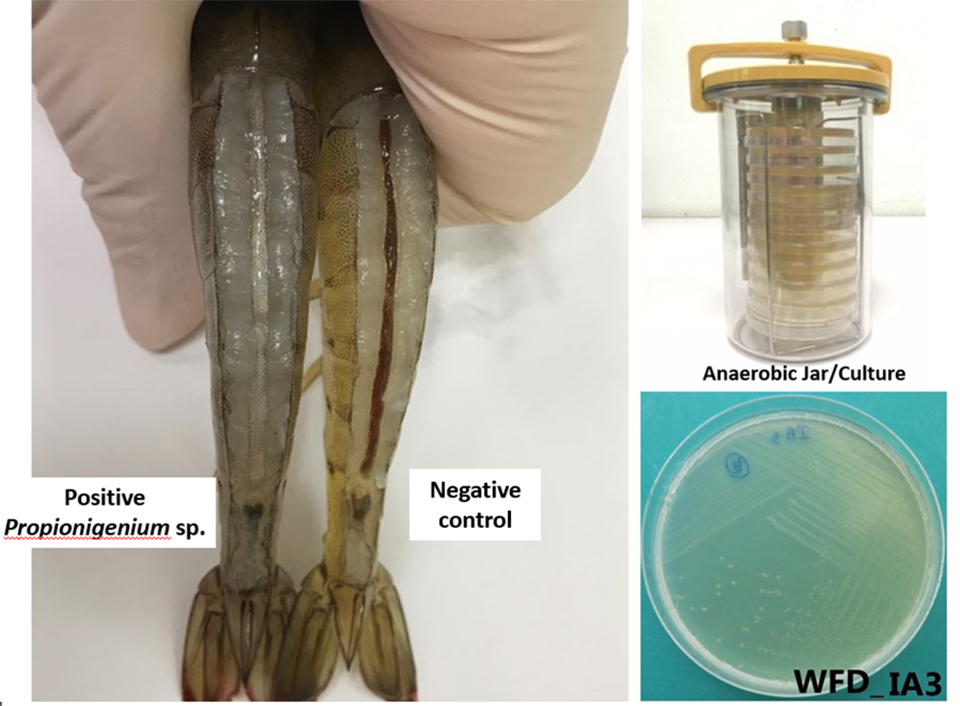
Now, at the same time we’re working with isolating anaerobic bacteria, not knowing what we’re doing, Dr. Kallaya from Mahidol University in Thailand had collected white feces from several areas in Thailand. Dr. Kallaya performed a metagenomic blast (Basic Local Alignment Search Tool, BLAST, the most well-known and commonly used tool for DNA search and alignment) of those feces to identify the bacteria and the white feces. And the bacterium that she found common to all of the white feces shrimp was Propionigenium.
I have collected white feces in Guatemala, and also Propionigenium. It is certainly a major player in white feces. It is anaerobic and generally, you’re going to find it in ponds with some bad bottoms with some anaerobic areas, old feed piles, things that have anaerobic areas that are going to provide habitat for Propionigenium. When the bacterium or Vibrio strains get into a shrimp with EHP, it’s going to magnify the effect of that EHP and you’re going to go into white feces, which is a worse form of EHP.
The most important aspect from my perspective as a shrimp breeder is to make sure our broodstock are not carrying Propionigenium. Because if our broodstock carry this bacterium to the hatchery, they’re going to pass it to the PLs. Now, maybe the PLs do not have EHP, so there’s no effect. But if PLs carrying Propionigenium get into a pond with EHP, now we got white feces, and it’s much worse. So, this is another screen that hatcheries could do to ensure that their hatcheries are not spreading this bacterium around.
There is EHP in the Americas at low levels, but it doesn’t seem to cause the disease. It has been found in many of the shrimp farming countries in the Americas. Several countries have been mentioned, but EHP has not been publicized mostly because there’s no disease associated with it. It is present at low frequency in the HP, and it doesn’t magnify in the HP, it’s low frequency. Is it a different EHP? We did take EHP from Guatemala and I sent it to be sequenced and it was the same as Asian EHP. At least from Guatemala, it’s no different.
Now why does it cause massive disease in Asia and not in the Americas? That, to me, was a key question to ask in trying to understand EHP. And I concluded that it’s all about stress and stocking densities. So let’s look at the case of Guatemala, which is an “intensive” shrimp farming country in the Americas and it’s got significant EHP issues, same as Asia.
So, the broodstock is American and the EHP and the intensive culture system is Asian. Their disease is the same. On the other hand, if we have a semi-intensive or low-density situation, you may have low-frequency EHP, probably not detectable and there’s no disease but the pathogen is present. If we took those animals and put them in a high-density culture system, the results would be Asian EHP disease. So that led me to believe stress is a major player here.
Let’s go back to a few other stories. This was probably maybe 2016 and EHP is creating many problems in Thailand. I’m going out to look at a farm with EHP problems. I’m having breakfast and looking at a neighboring farm on the same tidal creek. They’re doing great, their performance is spectacular, whereas another farm very close to it is a disaster.
So, I asked the manager of the first farm if they had EHP and he said yes, there’s EHP (the microsporidian) but there’s no disease. He added oxygen to ponds via mechanical aeration. This person happened to be an aerator supplier, so he had lots of aeration, and he kept his dissolved oxygen (DO) above 6 ppm. He told me if you keep the oxygen high, the animal is good, the environment is good, low stress for the shrimp even though there’s higher density, there’s lower stress because of the high levels of dissolved oxygen, and no EHP disease. So, I went to the other farm, the one with problems, and I check their DO and it is below 5 ppm and EHP had taken over. So, if I have lower oxygen levels, the EHP tends to take over because of the stress of lower DO.
There can be many stresses in a pond which makes the EHP infection worse and multiply in the shrimp HP. Low oxygen is one stress, but sulfides, high organics, nitrites, ammonia, carbon dioxide and others But it is true that higher stocking densities and higher feed rates will produce more stress in ponds and a higher severity of EHP in these ponds.
If we want to create a fast EHP response in the laboratory, we do EHP challenges. One of the things that we do as broodstock producers is try to create tolerance. EHP has been a real challenge for tolerance because it’s not a simple bioassay, it’s not a simple challenge test, it takes weeks and months.
How do you control the infection levels and other factors? It’s been very difficult. And the other question is, is there even such a thing as tolerance to EHP? I thought it was better to look at EHP, instead of a genetic solution, to come up with a management solution.
Now we could look at exclusion. Again, we’ve already noticed if it’s in the environment as it is in these countries, so when EHP gets into an area it starts off at low levels. Now maybe when it’s low level, we can control it much better, but as it builds up, meaning farm after farm get infected more and more and all the spores get spewed into the environment, the levels go up. Now the severity goes up and that’s why we have higher severity year after year. It starts out at X. Next year it’s X plus 10 percent. The year after it’s X plus 10 percent plus 10 percent more … it gets higher and higher as the infection levels in the environment increase.
Everything gets more difficult because, even if we are controlling stress levels in the shrimp, we’re overwhelming the organism with the pathogen. At that point, there has to be a way to bring the level of the EHP spores down, either through a production holiday or through cooperation, where we’re going to lower the stocking density levels and we make sure there’s no EHP going into the ponds. Hatcheries with EHP are mandated to be shut down, because as long as we’re feeding an environment with more and more spores, it becomes more and more difficult to reduce the stress.
I find that nitrite is a huge stressor for shrimp. So, if I add a little nitrite to my challenge, I can increase the rate of infection. You can really get a shrimp with high levels of EHP infection with a little nitrite in the culture. Stressors can be low oxygen, nitrites, pH fluctuations, sulfides, organic acids – whatever is an environmental stress is probably going to create the environment for EHP to take over a shrimp and become a very, very serious issue.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Darryl E. Jory, Ph.D.
Editor Emeritus
Tagged With
Related Posts

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Health & Welfare
How salinity levels impact EHP infection rates in Pacific white shrimp
An EHP infection can occur at a salinity as low as 2 ppt but the prevalence and the severity of the EHP infection is higher at 30 ppt salinity.

Health & Welfare
White Feces Syndrome in shrimp: Predictor of EHP?
Study demonstrates strong association between White Feces Syndrome and Enterocytozoon hepatopenaei in EHP-endemic regions. Biosecurity strategies can minimize the risk of pathogen’s spread in the Americas.

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

