Results show for the first time that shrimp fecal strings can be an infectious contamination route

The disease Hepatopancreatic Microsporidiosis (HPM), caused by the intracellular microsporidium Enterocytozoon hepatopenaei (EHP), has been reported in several penaeid shrimp, including black tiger shrimp (Penaeus monodon) and Pacific white shrimp (P. vannamei). EHP has been reported in different regions, including Asian countries such as China, Indonesia, Malaysia, Vietnam, Thailand and India. Recently, EHP has also been reported in Venezuela, located in the western hemisphere.
EHP causes lesions in the hepatopancreas (HP) tubule epithelial cells, and its main clinical signs are growth retardation that leads to increased size variability. Whitish fecal strings floating on the surface of pond water and the presence of shrimp displaying white discoloration of the gastrointestinal tract (GI tract) in these ponds have also been associated with EHP. In advanced stages of the disease, EHP-infected shrimp typically display soft shells, lethargy, reduced feed intake, an empty midgut and chronic mortalities.
In countries where EHP has been reported, such as India, China, Vietnam and Venezuela, shrimp farming is carried out in a wide range of environmental conditions, including coastal marine areas, estuarine areas and inland areas. For instance, in some states in the eastern part of India, such as Andhra Pradesh, the ponds are filled with borehole water mixed with estuarine water, causing the salinities of the grow-out ponds to fluctuate between 0 and 30 ppt with an average salinity of about 10 ppt. In contrast, in states located in the western part of the country such as Gujarat, salinities in pond water may vary between 30 and 44 ppt. In northwestern Venezuela, some shrimp farms are located around Lake Maracaibo, where the salinity ranges between 2 and 5 ppt, while in northeastern Venezuela shrimp farms are located in a marine environment where salinity ranges between 20 and 40 ppt, and EHP has been reported in both environments of high and low salinity. In both of these countries, the incidence of EHP seems to be higher in high salinity environments, but no study so far has assessed the possible relationship between salinity and the presence of EHP.
This article – adapted and summarized from the original [Aranguren Caro, L.F. et al. 2021. The effect of salinity on Enterocytozoon hepatopenaei infection in Penaeus vannamei under experimental conditions. BMC Vet Res 17, 65 (2021)] – reports on the results of a study to compare the infectivity of EHP using fecal strings as inoculum at three different salinities under experimental conditions.
Study setup
The bioassays were conducted in the Aquaculture Pathology Laboratory (UA-APL) of The University of Arizona. Specific Pathogen Free (SPF) P. vannamei were obtained from a commercial vendor in Florida, USA. The SPF population has been screened for the last two years at the UA-APL without the presence of any of the listed and non-listed diseases by the World Organization for Animal Health (OIE), including EHP.
The EHP isolate used in this study was obtained from a P. vannamei population originating in Thailand. Two independent EHP challenges were conducted. In both challenges, shrimp were maintained at three different salinities of 2 ppt, 15 ppt and 30 ppt. For each experimental challenge, six 90-liter tanks were filled with artificial seawater that corresponded to the three salinity levels with two replicates for each salinity treatment. Temperature was adjusted at 25 degrees-C (±0.6) with measurements each morning; pH was measured once a week with a range of 7.5–8.0.
Salinity was adjusted by changing three parts of the salinity every hour from 25 ppt (initial salinity of the SPF P. vannamei population) down to 5 ppt. In order to achieve a salinity of 2 ppt from 5 ppt, the salinity part was changed every two hours. Once set up, salinity was measured with a salt refractometer once a week over the length of the challenges. Ten (10) SPF P. vannamei (2.0–2.1 grams) were stocked in each tank for the experimental infection, respectively. Three 90-liter control tanks were set up for each salinity as negative control treatment.
For detailed information on the experimental design and animal husbandry; inoculum preparation; histopathology and polymerase chain reaction testing for detection and quantification of EHP; and statistical analyses, refer to the original publication.
Results and discussion
We investigated the prevalence and severity of EHP in three salinities: high (30 ppt), medium (15 ppt) and low (2 ppt) under laboratory-challenged conditions. The fecal strings used as a source of EHP inocula in the experimental challenges were sufficient to cause disease in shrimp maintained at different salinities, as confirmed by histopathology and PCR. The results from this study provide a new EHP infection method through fecal strings via the fecal-oral route.
The final survival at the end of the EHP challenge was high, ranging from 90 to 100 percent, in the three salinities in each of the two independent experimental challenges (Table 1). The survival percentages in the control treatments were 100 percent in all of the salinities in the two independent challenge experiments. We did not observe any clinical signs in the shrimp exposed to EHP in the 3 different salinities.
Aranguren, Salinity, Table 1
| Experimental Challenge | Duration | 2 ppt EHP treatment | 2 ppt control | 15 ppt EHP treatment | 15 ppt control | 30 ppt EHP treatment | 30 ppt control |
|---|---|---|---|---|---|---|---|
| 1 | 20 days | 95 ± 7 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 2 | 26 days | 90 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 90 ± 7 | 100 ± 0 |
Fecal strings were collected on a daily basis from known EHP-infected tanks. The daily fecal string samples tested positive for EHP in both challenges. The average weight of the fecal strings added to each tank was 1.17 ± 0.52 grams and 0.32 ± 0.24 grams for challenges 1 and 2, respectively. The EHP copy number in the fecal strings used as inoculum was significantly higher in challenge #2 (p < 0.05). EHP copy number in the fecal string in challenge 1 was 1.6 × 103 ± 2.1 × 103 copies /ng DNA compared to 1.1 × 106 ± 2.0 × 106 copies/ng DNA in the inoculum of challenge 2.
The prevalence and severity of EHP in the shrimp we experimentally challenged using fecal strings as inocula in the two independent challenges was assessed by H&E histology. In both experimental challenges, the fecal strings used as inocula were able to provoke the disease in SPF shrimp. The prevalence of EHP was 28.5 percent in challenge 1 vs. 50.0 percent in challenge 2. The data confirms that fecal strings are a reliable source of inoculum for experimental infection of EHP.
The prevalence of EHP at 2 ppt, 15 ppt and 30 ppt salinities in challenge 1 was 25, 33.3 and 25 percent, respectively. In challenge 2, the prevalence of EHP at 2 ppt, 15 ppt and 30 ppt was 33.3, 30.0 and 87.5 percent, respectively. The degree of severity was higher at a salinity of 30 ppt in the second experiment. In challenge 2, 50 percent of the EHP-infected population at 30 ppt displayed grade G3 (moderate to severe) and G4 (severe) lesions caused by the EHP infection. We found a strong association between salinities and EHP-infected shrimp, and the prevalence of EHP in shrimp exposed to high salinity (30 ppt) was higher than in shrimp exposed to low salinities (2 ppt and 15 ppt combined). The different grades of severity in this study are shown in Fig. 1.
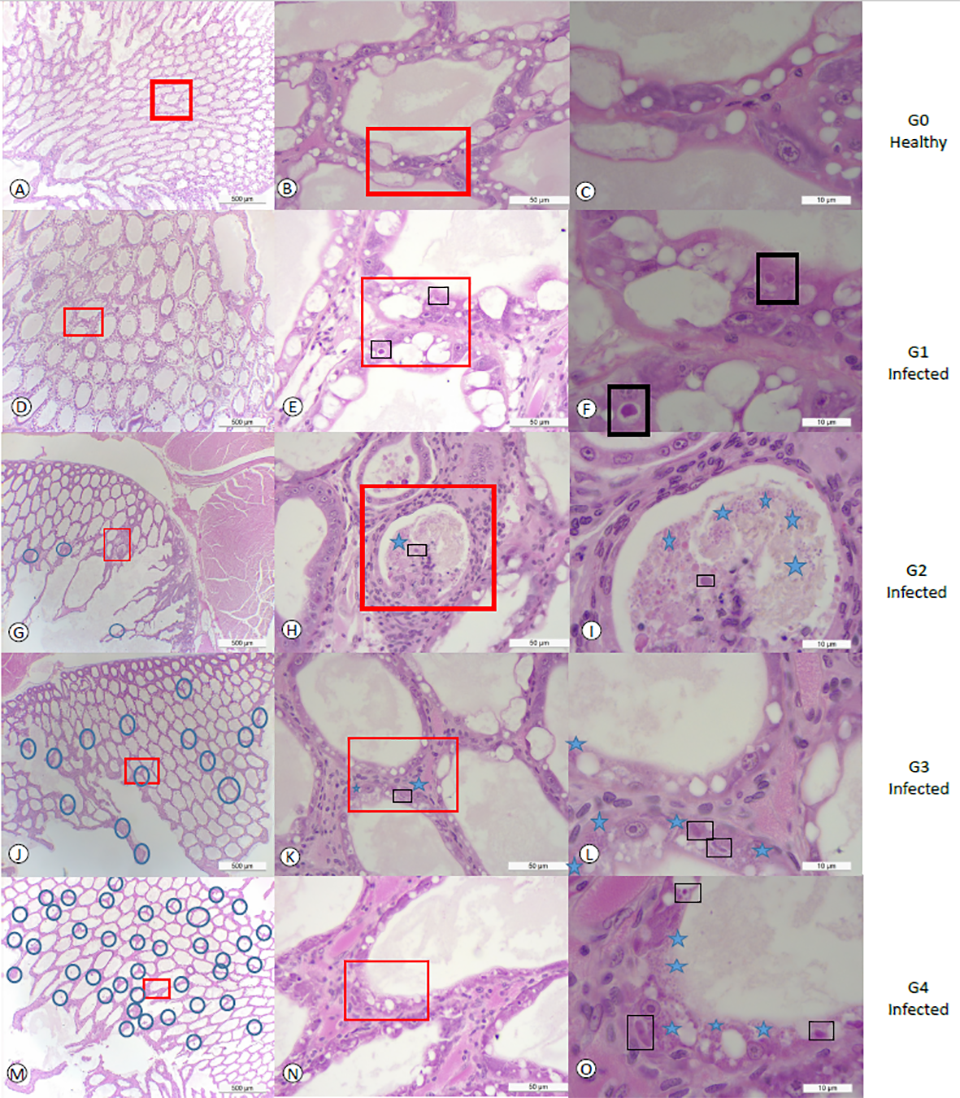
Fig. 1 panels A, B and C show tissue sections of HP at a low, medium and high magnification of healthy shrimp from the control tank without showing any histological lesions of EHP or any other known pathogens. In contrast, panels D, E, and F show HP tissue sections displaying grade G1 of an EHP infection. A focalized region within the HP was observed (Fig. 1d). The affected tubule shows the distinctive cytoplasmic inclusion bodies in the cytoplasm of the affected epithelial tubule cells that correspond to the uninucleate meront stage (Fig. 1e-f). Fig. 1 panels G, H and I show grade G2 of an EHP infection (low to moderate). The focal presence of an EHP infection in few affected HP tubules epithelial cells were observed. Both, the meront stage and spores liberated into the lumen were observed (Fig. 1i). Fig. 1 panels J, K, and L show a typical grade G3 of an EHP infection.
Multifocal lesions in HP tubules epithelial cells were observed (Fig. 1j). In the affected tubules, the presence of both irregular multinucleated plasmodium and spores within the cytoplasm of the cuticular epithelial cells were observed (Fig. 1). Fig. 1 panels M, N, and O show the grade G4 of an EHP infection with multifocal tubules containing infected HP cells (Fig. 1m). Both multinucleated plasmodium and spores within the cytoplasm of the affected cells as well as spores within the lumina of the tubules were observed (Fig. 1o).
The hepatopancreas of SPF shrimp challenged with fecal strings obtained from EHP-infected shrimp had positive results for EHP using nested PCR in all of the tanks at the three different salinities. This confirms the presence of EHP in the treatment tanks for challenge 1 and challenge 2. EHP was detected in all three salinities evaluated (2 ppt, 15 ppt, and 30 ppt). The hepatopancreas tissue collected from the negative control treatment animals reared at the 2 ppt, 15 ppt, and 30 ppt salinities tested negative for EHP using nested PCR.
Previous studies have shown some routes of infection included cohabitation, reverse gavage, and direct hepatopancreas injection with EHP-inoculum. In this study, we reported for the first time that feces can be an infectious contamination route. Due to the detritivorous behavior and the presence of undigested feed within the feces that could be around 25–30 percent, shrimp in treatment tanks were eating this source of food along with the EHP-infected spores mimicking the horizontal transmission observed at a farm level.
EHP lesions, including the presence of plasmodium in the cytoplasm of infected cells and we found the mature spores within the cytoplasm or released spores in the lumen by histopathology in EHP-challenged shrimp reared at different salinities. This unequivocally confirms EHP is able to cause an infection at a wide range of salinities, varying as much as 2 ppt to 30 ppt. When the initial inoculum used for the experimental challenge was low (i.e., 1 × 103 copies of EHP/ ng of total fecal DNA), the HPM prevalence was similar (i.e., 25 percent) irrespective of the salinities.
Determination of the infectious agent of Translucent Postlarva Disease in Pacific white shrimp
However, the prevalence of the EHP infection increased at 30 ppt salinity (87.5 percent prevalence), compared to 15 ppt (30.0 percent prevalence) and 2 ppt salinity (33.3 percent prevalence), when the inoculum level was increased from 1 × 103 to 1 × 106 copies of EHP/ ng of total fecal DNA in the challenge experiment (i.e., challenge 2). Dose-dependent challenge has been well documented for other shrimp pathogens such as AHPND and Hepatobacter penaei. In the present study, the inoculum with low copy number (1.6 × 103 copies of EHP/ ng of total fecal DNA) used in challenge #1 caused mild infections in the challenged shrimp. However, severe infections (Grades G3 to G4) and a higher prevalence occurred in challenge 2 when the EHP copy number in the inoculum was higher (1.1 × 106 copies of EHP/ ng of total fecal DNA).
The histological lesions in shrimp maintained at 30 ppt salinity were more severe. A moderate-to-severe grade of infection (G3-G4) was found in 50 percent of the shrimp affected. In contrast, only 16 percent of shrimp reared at salinity of 2 ppt showed grade G3 level of infection and 0 percent of shrimp reared at salinity of 15 ppt showed grade G2-G4 level of infection. The difference in the severity of the EHP infection at the three different salinities was probably due to the differential effect of salinity on spore germination. One of the critical phases in the spore germination is the increase of intra-spore osmotic pressure. The difference in salinities led to a hypotonic (lower concentration) environment at 2 ppt and 15 ppt compared to a hypertonic (higher concentration) environment at 30 ppt. It is possible that the hypertonic solution enhances the germination of the spore by increasing the spore activation process.
Hardness is another variable that was different in the three salinities used in this study and could have been a factor that affected the spore germination. The hardness in low salinity (2 ppt) was about 240 mg/L vs. the marine water artificially prepared at 15 ppt and 30 ppt that was around 787 and 1,575 mg/L, respectively. It has been reported that calcium is an important second messenger that activates many cell events and calcium influx might be, in part, responsible for the activation of microsporidian spore discharge at higher salinities.
In grow-out ponds of some EHP endemic areas in Asia, the salinity conditions are found to vary widely. For example, in India there are some shrimp farming areas in high and low salinity, and the prevalence of EHP seems to be lower at lower salinities (below 5 ppt), as observed during a shrimp disease survey in Andhra Pradesh in 2019. Similar conditions were recorded in two major shrimp farming areas in Venezuela, i.e., Maracaibo Lake, where the salinity is around 4–6 ppt and in Falcon state where the salinity varies from 36 to 40 ppt. In Venezuela, shrimp farming is not fully integrated, and the movement of nauplii and postlarvae between Falcon and the Maracaibo Lake area is a common practice. This suggests that EHP-infected PLs and/or broodstock have been moved between these two zones. However, EHP has only been detected in the Falcon area where the salinities are high. In Maracaibo Lake, where salinity levels are low, EHP has not been reported. One possibility that has limited the spread of EHP could be the difference in water salinity.
Conclusion
Results of our study demonstrated that fecal strings from known EHP-infected shrimp could be used as a reliable source of inoculum to conduct EHP experimental infections via the fecal-oral route. An EHP infection can occur at low salinity (i.e., 2 ppt) although the prevalence and the severity of infection is higher at a salinity of 30 ppt. These findings have implications in disease management in EHP-endemic areas.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Luis Fernando Aranguren Caro, Ph.D.
Corresponding author
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA[117,100,101,46,97,110,111,122,105,114,97,64,117,103,110,97,114,97,102,108]
-
F. Alghamdi
Health & Fisheries Services Ministry of Environment, Water and Agriculture, 65 King Abdulaziz Road, Riyadh, 11195, Kingdom of Saudi Arabia
-
K. De Belder
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA
-
J. Lin
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA
-
H.N. Mai
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA
-
J. Millabas
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA
-
Y. Alrehaili
Health & Fisheries Services Ministry of Environment, Water and Agriculture, 65 King Abdulaziz Road, Riyadh, 11195, Kingdom of Saudi Arabia
-
A. Alazwari
Health & Fisheries Services Ministry of Environment, Water and Agriculture, 65 King Abdulaziz Road, Riyadh, 11195, Kingdom of Saudi Arabia
-
S. Algetham
Health & Fisheries Services Ministry of Environment, Water and Agriculture, 65 King Abdulaziz Road, Riyadh, 11195, Kingdom of Saudi Arabia
-

Arun K. Dhar, Ph.D.
Aquaculture Pathology Laboratory, School of Animal and Comparative Biomedical Sciences, The University of Arizona, Tucson, AZ 85721 USA
Tagged With
Related Posts

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Intelligence
10 takeaways from GOAL 2019 in Chennai, India
The Global Aquaculture Alliance held its GOAL conference in Chennai, India, and recruited a host of experts in various fields to share their expertise.

Health & Welfare
New management tools for EHP in penaeid shrimp
Authors examined the histological features from shrimp infected with the emerging microsporidian parasite Enterocytozoon hepatopenaei (EHP). A PCR assay method was used to detected in hepatopancreatic tissue, feces and water sampled from infected shrimp tanks, and in some samples of Artemia biomass.

Health & Welfare
New nested PCR test targets gene specific to farmed shrimp pathogen EHP
A new nested PCR method specific to farmed shrimp pathogen EHP can be used in routine diagnostics and is also useful to detect low-grade EHP infection.



