Evaluating the reproduction, culture parameters of potential species
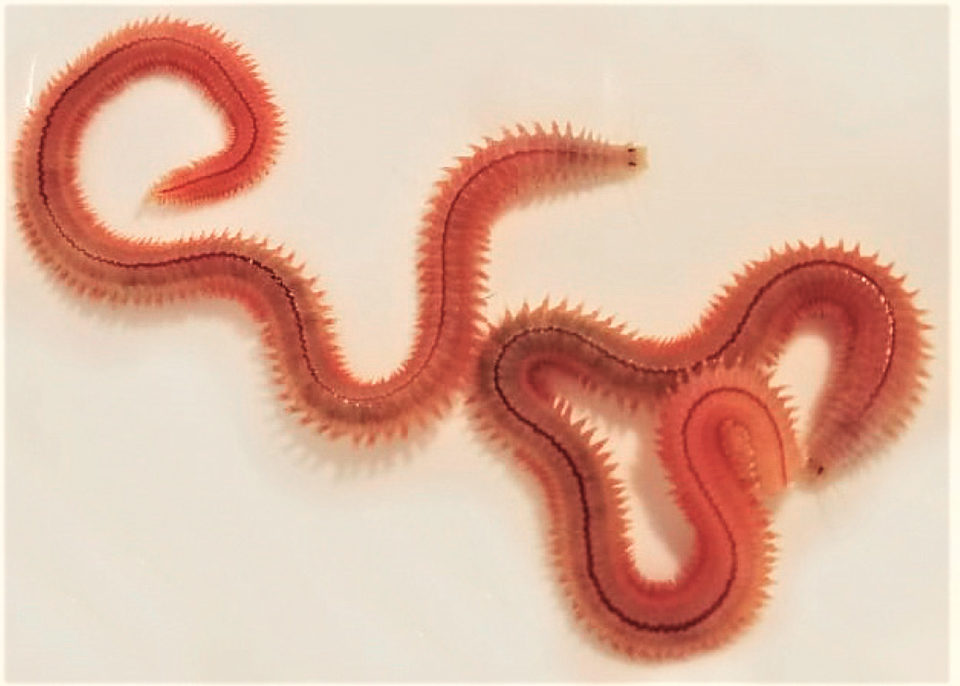
The use of live polychaetes for the maturation phase of penaeid shrimp broodstock improves the quality and quantity of shrimp nauplii (shrimp larval; stage) produced. The genera Australonuphis sp. and Perinereis sp. have been used as maturation feeds for shrimp broodstock in America and Asia, respectively, and many studies specifically report the benefits of using the latter in shrimp maturation, especially for Pacific white shrimp (Litopenaeus vannamei) in Asian shrimp farming countries.
Shrimp maturation laboratories in various parts of the world use polychaetes captured in the natural environment, but – due to biosecurity concerns – this practice implies significant biological risks for the industry. A source of quality maturation feeds that are effective in improving shrimp reproduction and also free of biological risks is needed.
We initially surveyed and identified native polychaetes on the coast of Ecuador (April 2019) and concluded that the genera Australonuphis, Lumbrineris and Perinereis are good candidates to study regarding their reproductive biology and culture parameters. In this article – adapted and summarized from the original (Revista Acuacultura-Cámara Nacional de Acuacultura, No. 129, June 2019) – we carried out a study to select an appropriate polychaete genus suitable for starting the production of live, disease-free animals for the maturation of shrimp broodstock in Ecuador.
Study setup
Husbandry
The study of the reproductive biology and culture parameters result in the ability to maintain optimal production conditions for the cultured species, so we designed a water recirculation system (RAS) that guarantees the necessary water quality through the use of mechanical and biological filtration. The system consists of four tanks where the different species of polychaetes to investigate are stocked, cultured and studied. Seawater is adjusted to a constant salinity and temperature, and a daily record of several parameters – pH, alkalinity, ammonium, nitrites and nitrates – parameters has been maintained.
The ease of handling of the animals is a key aspect to culture large numbers of polychaetes, and we have evaluated whether the animals can escape from their tanks, if they burrow into the bottom sediment, and if they can withstand handling without suffering physical damage when they are manipulated during substrate cleaning operations and during harvesting.
Polychaete cultivation will only be successful if the animals accept the food provided to culture them. We have evaluated the acceptance of two types of feeds – fresh and dry diets – by the different polychaetes, and the acceptance of dry feed will facilitate maintaining specific-pathogen-free (SPF) conditions of the polychaete production facility.
Reproduction
We have made observations to detect the presence of breeding animals in the culture system, using both a stereo microscope (eyepieces 10x and 20x; objectives 2x and 4x) and an optical microscope (eyepieces 10x and 20x; objectives 4x, 10x, 20x and 100x).
Once the broodstock animals for each polychaete genus had been identified, we evaluated their maturation state by observing the eggs and sperm of these specimens through the tegument (outer body covering). Subsequently, samples of eggs and sperm were obtained to assess their maturation status.
The spherical shape and a certain egg size signal the optimal condition to be fertilized. In the case of sperm, the optimal status indicator is given by the sperm motility. Once mature individuals in optimal state for fertilization have been identified, the specific fertilization protocols for each of the polychaete genera we investigated were implemented, with the ultimate goal to standardize the fertilization protocols and to ensure that polychaete larvae are obtained through reliable procedures that can be replicated.
Results and discussion
The maintenance of specimens of Australonuphis sp. resulted in problems with the survival of adult individuals for prolonged times in the RAS system, although younger individuals appear to be more resistant and adaptable. Despite their high mortality in the culture system, individuals are contained in the culture tanks and bury in the bottom sediments, and no escapes have been observed. On the other hand, Lumbrineris sp. and Perinereis sp. individuals have better adapted to the culture environment, bury themselves in the sediment with ease, do not escape from the tanks and survive for several weeks.
Ease of handling of the cultured animals is a fundamental issue when deciding on the production of one species or another. Australonuphis sp. has presented many problems in this regard because the manipulation by personnel has caused stress to, and spontaneous break-up of the animals. In the case of Lumbrineris sp. and of Perinereis sp., handling has been possible because the animals are more resistant. This indicates that the harvest of commercial size individuals from these two genera of polychaetes could be carried out without problems, potentially making their cultivation attractive.
We have observed that the genera Australonuphis sp. and Lumbrineris sp. are not attracted to fresh or dried feeds. However, Perinereis sp. individuals captured on the island of Jambelí have shown attraction from the first day in the cultured system for both types of feeds, and this has allowed us to maintain these animals for a long time in healthy conditions.
Some Australonuphis sp. individuals, when freshly caught, exhibited the typical characteristics of mature specimens. Some females have been observed with eggs, identifiable by the greenish coloration on the rear half of their body, or pink-whitish color in the case of males. Both eggs and sperm have been observed for transparency through the tegument of the animals.
We have found developing eggs (30 to 100 μm diameter) in Australonuphis sp. females, and more advanced maturation eggs (220 to 240 μm diameter) in other individuals. Developed and mobile sperm have also been observed in specimens of Australonuphis sp. (Fig. 1).
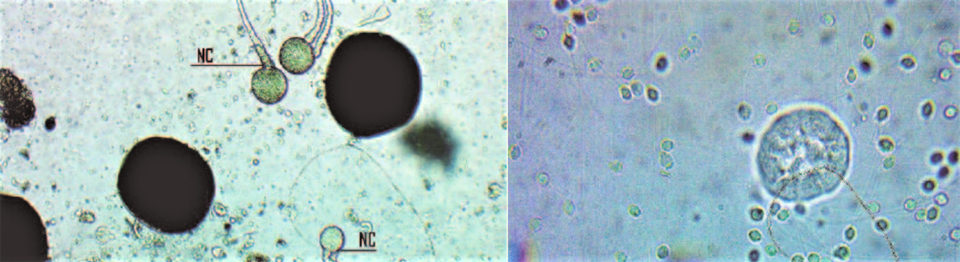
In the case of Lumbrineris sp., no mature individuals were found at the collection points. However, after a few weeks in the culture system, a female developed a large number of eggs (Fig. 2). No attempt at fertilization could be made, since no mature males have been observed yet in the RAS system tanks.
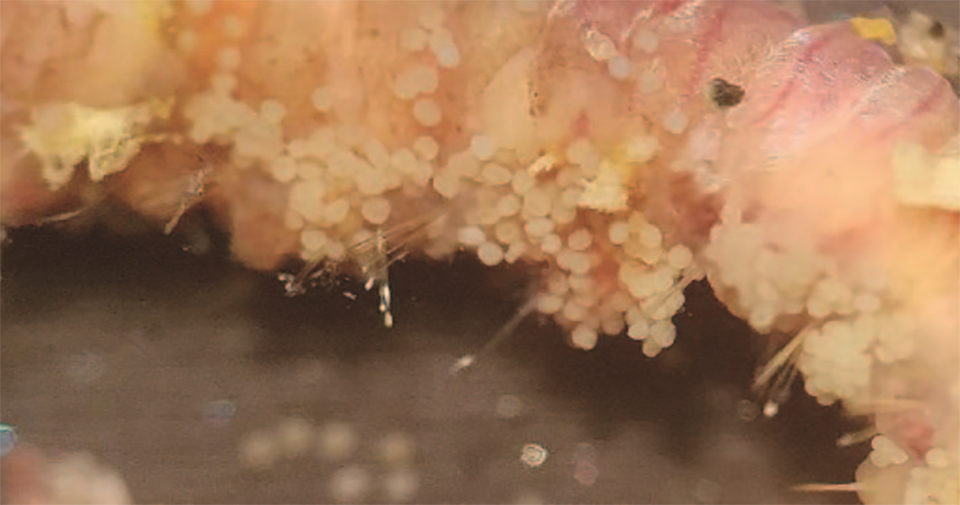
Some specimens of Perinereis sp. have developed eggs and sperm under culture conditions. These individuals initiated the morphological transformation that characterizes many of these polychaetes, which occurs prior to their reproduction, with some individuals transforming and developing enlarged eyes, flatter morphology and a shortening of the body length. The mature females had a greenish color (due to the presence of eggs inside) and the males a pinkish-white color due to the sperm contained in the coelom (body cavity).
We have achieved the in vitro fertilization of several hundred eggs of Australonuphis sp. applying polychaete reproduction techniques, and relevant observations on fertilization and larval development are noted in Table 1. We noticed that the first mitotic division occurs within 60 minutes after the start of the egg-sperm contact time under temperature conditions and water salinity designed for fertilization of this species.
Naranjo, polychates, Table 1
| Day (hours) | Length (μm) | Appearance | Setigers |
|---|---|---|---|
| Start | 240 | Spheric eggs (fertilization) | 0 |
| Day 0 (1 hr.) | 240 | Two cells (first mitotic division) | 0 |
| Day 0 (7hr.) | 240 | Embryos with rotational movement | 0 |
| Day 4 | 640 | Larva with differentiated structures (eyes, jaws, setae) | 5 |
| Day 5 | 680 | Larva with differentiated structures (eyes, jaws, setae) | 5 |
| Day 6 | 755 | Larva with differentiated structures (eyes, jaws, setae) | 6 |
After seven hours from the first division, developing embryos are observed that swim in the water column with a rotational movement. During the following days it was observed that the embryos transformed into pyriform-like larvae that were swimming toward the bottom of the culture containers. After four days and beyond, vermiform (worm-like) larvae could be observed that swam very close to the bottom of the containers, and even walked along above the surface. These larvae already had distinguishing structures such as eyes, jaws and setae (bristle- or hair-like structures) used to move.
We have separated individuals of Perinereis sp. with eggs and sperm inside (epitocals) from the rest of the specimens, to work on their fertilization, and have obtained hundreds of larvae that develop following the scheme shown in Table 2. After 60 minutes from the beginning of the egg-sperm contact, the first mitotic division could be observed. At 24 hours, several rotating embryos were observed inside an embryonic capsule. After three to five days, larvae with a benthic behavior and three to five setigers (segments with bristles) were observed, which also had well differentiable structures such as eyes, antennae and anal structures.
Naranjo, polychates, Table 2
| Day (hours/months) | Length (μm / mm / cm) | Appearance | Setigers |
|---|---|---|---|
| Start | 250-300 μm | Spheric eggs (fertilization) | 0 |
| 1 hr. | 250-300 μm | Two cells (first mitotic division) | 0 |
| Day 1 | 300 μm | Embryos with rotational movement | 0 |
| Days 3-5 | 350-500 μm | Benthic larvae in development | 3-5 |
| Day 23 | 2 mm | Juvenile specimens with differentiated structures (eyes, jaws, palps, antennae and quetas) | 12 |
| Month 5 | 8-15 cm | Animals in culture / adults | several tens |
The Perinereis sp. juvenile specimens obtained in these first fertilizations had a length of 2 mm after 23 days, 12 setigers and structures such as eyes, jaws, antennae and anal structures (Fig. 3). After five months, the individuals had achieved a length of 8 to 15 cm (Fig. 4).
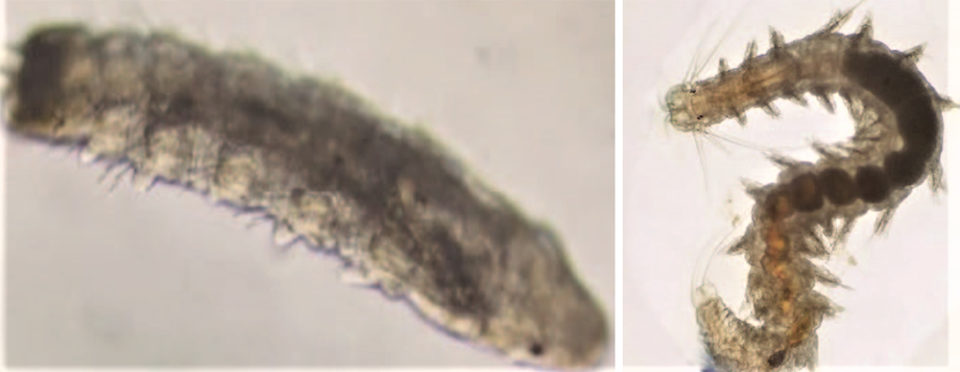
Following the first fertilization, more specimens have been obtained using the same procedure. From this milestone, individuals are expected to reach adulthood and begin to mature and to reproduce. The objective is to achieve a critical mass of breeding individuals that can support a commercial culture operation.

Perspectives
The Australonuphis sp. and Lumbrineris sp. we studied could be good culture candidates based on what we now know about their reproduction and husbandry. However, the fragility of Australonuphis sp. and the lack of data on larval fertilization and development of the native population of Lumbrineris sp. suggest the culture of Perinereis sp. as a better alternative because of its handling characteristics, food acceptance and reproduction.
The next objective is to start the production of Perinereis sp., and we have designed a pilot plant where the first generations of individuals will be raised. Once a critical mass of broodstock animals is reached, we will begin industrial-scale production under SPF conditions.
References available from first author.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Andrea Paola Naranjo
Corresponding author
Empresa APRACOM, S.A.
Guayaquil, Ecuador[109,111,99,46,99,101,45,109,111,99,97,114,112,97,64,111,106,110,97,114,97,110,112]
-
Francisco Javier Tobías
CEAB-CSIC, Consejo Superior de Investigaciones Científicas
Madrid, Spain
Related Posts

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 2
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors considered biotic and abiotic factors. Part 2 describes results of their study.

Responsibility
Polychaete-assisted sand filters show potential in treating effluents
A practical way to treat mariculture effluents combines the physical attributes of a sand filter and the biological properties of hungry marine sand worms.

Aquafeeds
Polychaete worms reduce waste, provide food in aquaculture
Adaptable and diverse, polychaetes can adapt their feeding behaviors to environmental conditions. Nereis diversicolor, a marine polychaete that can tolerate wide temperature and salinity ranges, is a good candidate for RAS enhancement.

Aquafeeds
Biosecurity protocols needed for shrimp feeds, feeding practices
Shrimp aquafeeds – live, fresh or formulated – should not be an entry point of potential pathogens to the shrimp and/or to their culture systems.



