Study demonstrates the potential of a nanoparticle platform to deliver dsRNA antivirals to aquatic animals

The White Spot Syndrome Virus (WSSV) is the most economically and globally significant shrimp pathogen, destroying a tenth of farmed shrimp production (about U.S. $1 billion) every year. Shrimp infected with WSSV succumb to the pathogen within two to seven days, with mortality as high as 100 percent. Developing disease intervention approaches that are safe and efficacious is important, and in this regard, vaccination strategies such as inactivated virus, subunit antigen, and DNA-based vaccines against WSSV have shown promise at the laboratory scale. However, the drawbacks – such as variable efficacy, high manufacturing cost and limited field applicability – warrant further research.
RNA interference (RNAi) in invertebrates is an antiviral cellular mechanism by which a trigger, such as double-stranded RNA (dsRNA) or small interfering RNA (siRNA) prevents viral gene expression. These dsRNA have been tested in a broad variety of settings, such as in plant pest control, next-generation mosquitocides and vaccine design. In aquaculture systems, the concept of RNAi-based vaccines has been championed for several reasons: (a) RNAi works as an antiviral immune response in shrimp; (b) it is pathogen-specific; and (c) it generates a long-term protective immune response.
Previous work has shown that dsRNA-based vaccines are nontoxic, efficacious and provide protection against a lethal dose of WSSV. However, in all the applications mentioned above, the main challenge for the progression of a dsRNA-based vaccine from discovery to product and field application is its environmental stability and manufacturing costs. We hypothesize that the limitations to dsRNA delivery in the field could be overcome using a nanocarrier delivery platform that further enhances cellular uptake, establishes long tissue residence times, is dose-sparing thus reducing the cost, and is adaptable for mass vaccinations. In this respect, biodegradable polyanhydride nanoparticles have proven to be efficacious delivery systems for antigen-based vaccines and drugs.
This article – adapted and summarized from the original publication (Phanse, Y. et al. 2022. RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp. Vaccines 2022, 10(9), 1428) – discusses the results of a proof-of-concept study, the first one to the authors’ knowledge and combining two platform technologies (RNAi-based virus-specific dsRNA and a polyanhydride nanoparticle-based delivery platform) to develop a nanovaccine for aquaculture applications.
Study setup
Specific-pathogen free (SPF) L. vannamei were procured from Shrimp Improvement Systems (Islamorada, Fla., USA). Animals were acclimated for one to two weeks in 1-ton aerated fiberglass tanks filled with artificial seawater (salinity 28–30 ppt). Water temperatures were maintained at 25–27 degrees-C. The animals were fed twice a day with Raceway plus commercial shrimp feed according to size and developmental stage (Zeigler Bros, Gardners, Pa., USA).
The effect of nanoparticle chemistry on safety, biodistribution and persistence was evaluated in vivo in the shrimp. To mimic an oral route of administration, nanovaccines were injected via reverse gavage to target the gastrointestinal epithelia, and the nanovaccine efficacy was tested in a lethal WSSV challenge model in vivo in shrimp.
For detailed information on the experimental design; dsRNA synthesis; polyanhydride and nanoparticle synthesis; histopathology, biodistribution and other study components; and statistical analyses, consult the original publication.
Results and discussion
Even though nanomedicine has already made notable contributions to human health, its use in promoting animal health is still in its infancy. Numerous studies have shown that polyanhydride nanoparticles are excellent vehicles for antigen delivery by providing antigen stability, controlled release, intrinsic support and protective immunity. However, this is the first study to our knowledge demonstrating their use as dsRNA delivery vehicles for prophylactic vaccination strategies in invertebrates.
Polyanhydride nanoparticles were shown to be suitable for the encapsulation and release of dsRNA. While both nanoformulations we tested provided near-zero order dsRNA release kinetics, the encapsulation efficiency of dsRNA was much greater in one, referred to as 20:80 CPTEG:CPH nanoparticles.
One of the foremost concerns for feed animal vaccines is that they should not cause any adverse effects to the final yield. Our data demonstrate that neither of the two nanoformulations tested induced any untoward effects in shrimp injected via reverse gavage, even with a dose of nanoparticles that was five-fold higher than that required for vaccine delivery. Blinded histopathological analysis confirmed that no histological abnormalities were caused in the shrimp alimentary tract tissue or gills due to nanoparticles. And a marker for inflammation in shrimp was also evaluated and found to be no different than that in control animals. Finally, shrimp weight gain, which is the most important contributor to the farm yield, showed that particles did not affect the normal shrimp development. These results are in accordance with previous work demonstrating the safety of polyanhydride nanoparticles.
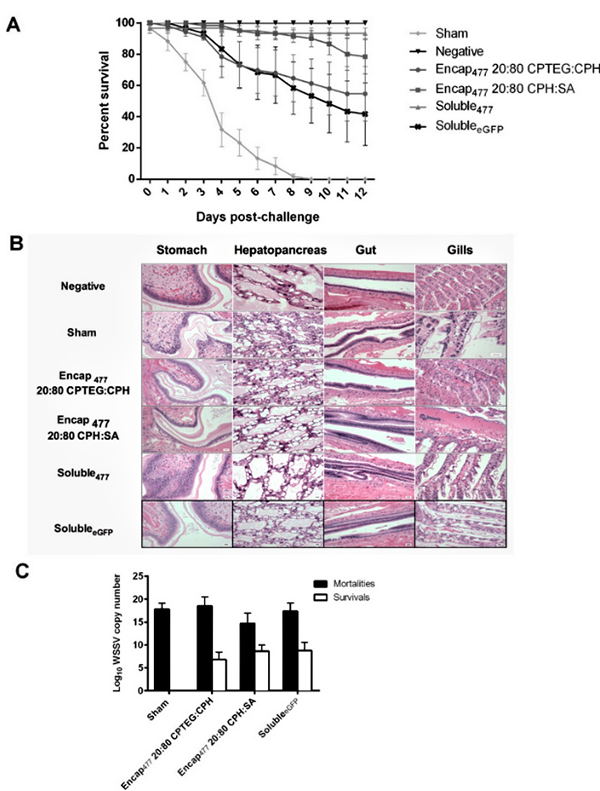
We also tested if the nanoparticle chemistry impacts tissue biodistribution and persistence in L. vannamei. As expected, nanoparticles were present in the gut, no doubt facilitated by the route of administration; however, within two hours they were also visualized in the stomach, hepatopancreas and cephalothorax. Interestingly, at day 0, gill tissues showed very high levels of the nanoparticles. While our previous work has demonstrated that the particles migrate systemically in other animal models, the mechanism(s) by which the nanoparticles trafficked to the gills remains to be determined. It is important to note that gills are one of the target organs for several viral pathogens such as Taura Syndrome Virus, Yellowhead Virus and WSSV. Thus, the presence of nanoparticles in gills potentially contributes to protection against diseases caused by these viruses.
Nanoparticle persistence is another important outcome to enable vaccinated animals to be protected from the disease through different life stages (post-larva, juvenile, or adult). Our previous work has shown that some polymer properties can significantly affect the tissue residence time of nanoparticles in mice. Both of our nanoparticles, CPH:SA and CPTEG:CPH, persisted in shrimp alimentary tract tissue and gills for at least 21 days post-injection. The combination of long persistence of particles along with short life span of shrimp may compensate for the lack of adaptive immunological memory and provide protection during the grow-out phase of shrimp farming.
Vaccines based on dsRNA can be effectively used against several shrimp viruses; however, development of suitable delivery strategies for mass vaccination of shrimp in aquatic systems is a major challenge. Oral administration to shrimp (i.e., through feed) is by far the most appealing method of vaccine delivery. Our experiments demonstrated that our nanovaccine, when administered directly to the gut, provided significant protection against a lethal dose of WSSV. A reverse gavage strategy for administration was used to mimic oral delivery and the results indicated that the nanovaccines protected encapsulated dsRNA from the gut environment and delivered a stable and functional WSSV RNAi trigger. The nanovaccines not only increased the survivability of challenged shrimp but also reduced WSSV replication in vivo.
This finding can have important implications in the field, as it is known that horizontal transmission of WSSV in a shrimp pond can either be by direct contact of infected cadaver, i.e., by cannibalism or by indirect water-borne route. Regardless of the route of transmission, the reduced virus load may thus contribute to abating the spread of WSSV in field conditions by lowering the available viral dose.
Although one of our nanovaccines provided greater protection against infection in this work than the other, it is important to understand the advantage of each nanoformulation. For example, one of the nanovaccines resulted in a large initial burst, such that almost 90 percent of the encapsulated dsRNA was released rapidly. Due to this large burst, high amounts of dsRNA were likely available in the shrimp alimentary tract at the time of infection (72 hours post-vaccination) and enhanced protection. In contrast, the initial burst of dsRNA from the other nanovaccine was lower. Overall, it is conceivable that administering a cocktail of both nanovaccines will enable both early and long-lasting immunity in the shrimp.
Perspectives
Altogether, the data from our study demonstrate the ability of the polyanhydride nanoparticle delivery platform to deliver dsRNA antivirals to aquatic animals. WSSV, being the most threatening of the shrimp viruses, was used in this study; however, the rapid tailoring of dsRNA against other viruses can make this adaptable to other diseases too.
Moreover, the versatility offered by this technology to deliver a wide range of antibiotic, antiviral and antifungal molecules has the potential of improving global aquaculture by disease control. The end result is a novel product that ultimately provides more effective, lower-dose therapies to benefit animal health, with tremendous potential to thereby contribute to both enhanced food safety and security and environmental health.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Lyric C. Bartholomay, Ph.D.
Corresponding author
Department of Entomology, Iowa State University, Ames, IA 50011, USA; and
Department of Pathobiological Sciences, University of Wisconsin-Madison, Madison, WI 53706 USA[117,100,101,46,99,115,105,119,64,121,97,109,111,108,111,104,116,114,97,98,108]
Tagged With
Related Posts
Health & Welfare
Alphavirus replicon particles potential method for WSSV vaccination of white shrimp
A study demonstrated that VP19 and VP28 white spot syndrome virus envelope proteins expressed by replicon particles provided protection against mortality due to WSSV in shrimp.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
CENIACUA develops WSSV-resistant shrimp in Colombia
To combat white spot syndrome virus (WSSV) in white shrimp, Corporación Centro de Investigación de la Acuacultura de Colombia (CENIACUA) initiated a selective-breeding program to develop resistance in shrimp.

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.



