Microencapsulation of Bacillus subtilis E20 proved effective in addressing probiotic issues related to inclusion during feed manufacturing

Several studies have reported the benefits of including probiotics in shrimp diets and modifying the bacterial profile of the shrimp intestine. Despite the benefits reported, it is believed that the direct administration of live probiotics reduces their cell viability, undermining the full potential of the probiotic. In particular, the sensitivity of probiotic bacteria to heat limits its application in the shrimp feed manufacturing processes, which use high temperatures.
Encapsulation techniques such as spray drying, freeze drying, and electrodynamics are deemed effective strategies to permit high viability and provide a high degree of protection against processing, storage and gastrointestinal conditions. These techniques control the release of probiotics in the intestine to exert modulatory effects on gut microbiota.
The application of next-generation sequencing (NGS; a technology for determining the sequence of DNA or RNA to study genetic variations associated with various biological phenomena; it allows the sequencing of many DNA strands at the same time, instead of one at a time as with traditional sequencing) techniques for shrimp can help elucidate shrimp-bacteria interaction. To date, no studies have specifically addressed the effects of encapsulated probiotics on the composition, diversity and function of microbiota in shrimp.
This article – summarized from the original publication (Cheng, A-C. et al. 2023. Microencapsulation of Bacillus subtilis E20 Probiotic, a Promising Approach for the Enrichment of Intestinal Microbiome in White Shrimp, Penaeus vannamei. Fishes 2023, 8(5), 264) – reports on a study that analyzed the microbiota associated with the intestine of encapsulated probiotic-fed Pacific white shrimp (Litopenaeus vannamei) and unencapsulated-fed shrimp using Next-Generation Sequencing (NGS) of 16S ribosomal RNA (16S rRNA; it is the most widely used marker gene in microbial ecology).
Study setup
L. vannamei shrimp were obtained from the Department of Aquaculture at the National Pingtung University of Science and Technology, in Pingtung, Taiwan. Before the study, shrimp at intermolt stage were acclimated for seven days in 10-cubic-meter cement tanks holding 5 tons of seawater at 20 ppt salinity and air stones for aeration. Then, 200 juvenile shrimp (1.89 ± 0.06, mean ± SE), with all appendages in good condition, were distributed into two cement tanks (6 × 2 × 1 m).
Shrimp were allocated to two dietary treatments (n = 100 each), one being a control and the other being the encapsulated probiotic, B. subtilis E20 (EP). The probiotic B. subtilis E20 was encapsulated in alginate-chitosan bilayer microparticles following a published procedure. Diet formulations using the encapsulated probiotic at 107 CFU/kg (EP), and a basal control diet was prepared and fed to shrimp for 60 days. The experimental diets were prepared based on our previous study’s diet with the highest growth performance and improved health status.
For detailed information on the experimental design, animal husbandry, data collection, intestinal microbiota analysis using Next-Generation Sequencing, and biodiversity and abundances of intestinal microbiota determinations, refer to the original publication.
Results and discussion
The shrimp intestinal microbiome consists of several microbes and genes critical for health, metabolism, as well as disease pathogenesis. As shrimp are intimately connected to the aquatic environment, much of their intestinal microbes are influenced by the microbes present in the surrounding environment. Consequently, culture systems that are either intensive or unfavorable adversely affect the microbial interaction between the shrimp and the environment, resulting in the proliferation of opportunistic pathogens that cause disease outbreaks.
Live probiotic bacteria – which are generally regarded as safe due to their immunomodulatory, antimicrobial, and antioxidant beneficial effects – are often incorporated into feeds as dietary supplements to maintain the microbial balance in shrimp gut. However, the viability of probiotics is greatly affected by numerous factors, especially during production, storage, feeding and passage through the gastrointestinal system. Several techniques for microencapsulation have been reported to preserve and protect the viability of probiotic cells
While most of these studies focused on the immune response and growth performance as well as intestinal microbiota upon live probiotic administration without encapsulation, studies on the intestinal microbiome upon administering encapsulated probiotics are limited.
In this study, the B. subtilis E20 strain was encapsulated with alginate-chitosan to protect cell viability and determine the bacterial communities generated. Data from NGS analysis revealed a dominant presence of Proteobacteria in all shrimp microbiota. Other researchers reported similar results when B. subtilis E20-fermented soybean meal (FSBM) was provided to shrimp.
These results suggest that microencapsulation of B. subtilis E20 (EP) can induce the proliferation and diversification of bacteria in shrimp microbiota.
The taxonomical analysis revealed that the majority of the bacterial genera were distributed among different families (193), with shrimp fed the control diet indicating a higher number of genera (275) than the shrimp fed the EP diet (236) (Fig. 1). Among the intestinal samples in the control and EP group, the shared bacterial genera were 89 and 67, respectively.
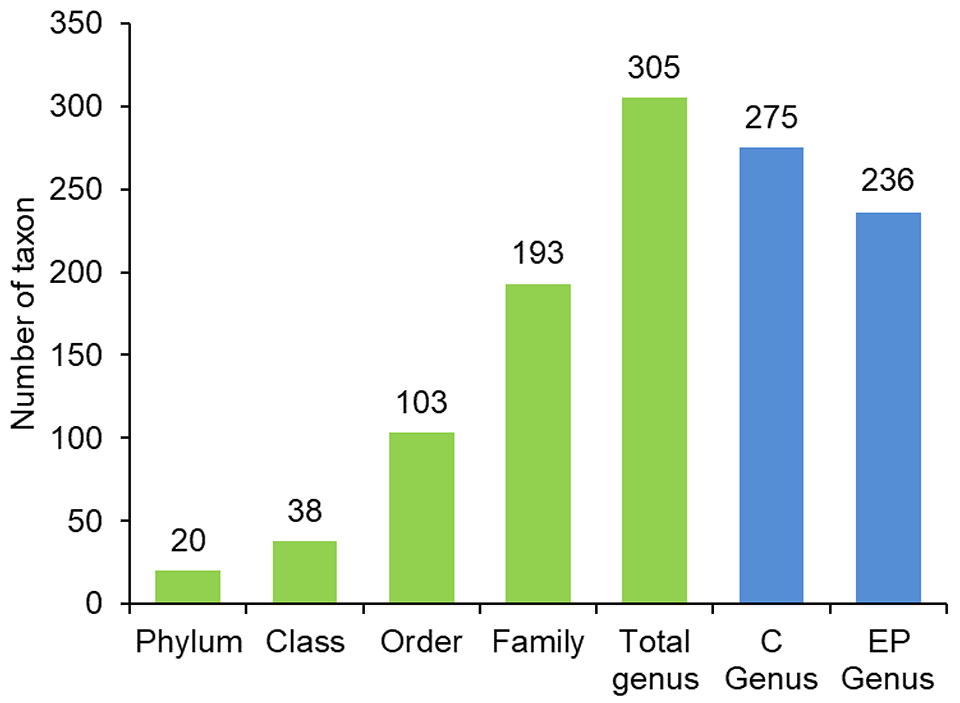
At the phylum level, the relative abundance of bacterial groups in the intestinal microbiota of shrimp fed the control and EP diet was predominantly Proteobacteria at 85.24 percent and 63.13 percent, respectively. The EP diet was further influenced by the phylum Tenericutes (12.96 percent), Bacteroidetes (10.80 percent) and Firmicutes (10.68 percent), all of which were minimally expressed in the control group. In shrimp fed the control diet, the most abundant at a generic level were Vibrio (70.74 percent), compared to the EP group, which had a lower Vibrio abundance of 30.25 percent.
Generally, the health condition of shrimp and fish can be reflected by the relative abundance of Proteobacteria, which is a microbial sign of dysbiosis (an imbalance of microbial species and a reduction in microbial diversity within certain body microbiomes) and disease in gut microbiota. Tenericutes are free-living organisms and exhibit metabolic and adaptivity flexibility commensal to the host. Firmicutes help ferment carbon sources and control energy balance within the host.
Similarly, Bacteroidetes ferment plant-derived substrates in the intestines by producing short-chain fatty acids (SCFAs) that allow the host to obtain excess energy; SCFAs also play major roles in the homeostasis of immune cells in several organisms. Therefore, the interaction between Firmicutes and Bacteroidetes likely promoted more efficient fermentation of carbohydrates in the diet and increased the energy absorption in the intestine of shrimp fed with encapsulated B. subtilis E20.
In addition, Vibrio species are among the dominant members of the L. vannamei shrimp microbiota and are considered the most important bacterial pathogens responsible for several diseases and mass mortalities. Several studies have reported the importance of Vibrio during the different developmental stages of shrimp. Vibrio populations in the shrimp gut microbiota are typically higher during the nursery stage than in the adult stage, indicating that the microbiota in the latter stage is more diverse than in the nursery stage.
Vibrio species are considered opportunistic pathogens that have detrimental effects on shrimp’s growth, metabolic activity, microbial balance and immune response. High expression in the gut is an indicator of disease in shrimp. For example, in infected shrimp, V. parahaemolyticus increases intestinal permeability and impairs the ability to absorb the amino acids and glucose that are necessary for maintaining physiological activities.
The supplementation of probiotics is a beneficial biocontrol agent for reducing Vibrio counts and preventing vibriosis. In this study, by analyzing the microbial community we determined that the Vibrio count and abundance levels of Vibrio species were suppressed in the intestine of shrimp fed the encapsulated B. subtilis E20 compared to shrimp fed the control diet.
The microencapsulated probiotic in our study also increased the abundance of Candidatus Bacilloplasma in shrimp. Candidatus Bacilloplasma are the dominant bacteria in the intestines of healthy shrimp, and a change in its abundance can contribute to shifting the intestinal microbial communities in shrimp suffering pathogens. Candidatus Bacilloplasma are recognized as symbionts (two organisms of different species that have a close and long-term biological interaction) and can be used as potential taxonomic indicators for assessing the health status of shrimp.
In previous studies, the detection of Candidatus Bacilloplasma showed commensal activities which inhibited the proliferation of Vibrio bacterial strains and infection. The greater expression of Candidatus Bacilloplasma in this study suggests that the encapsulation of probiotics can preserve their viability to such an extent that it stimulates the growth of various beneficial bacteria that might be lost when the probiotic is unencapsulated.
Perspectives
This study concludes that microencapsulation of B. subtilis E20 can be helpful in tackling the sensitivity problems associated with probiotics during processing and application. Our results indicate that encapsulated B. subtilis E20 administration increased beneficial strains of bacteria such as Bacillus and reduced the harmful bacteria belonging to the Vibrio species. Thus, the encapsulation of B. subtilis E20 has the potential to modulate gut microbiota and control Vibrio species in shrimp.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Chung-Hung Liu
Corresponding author
Department of Aquaculture, National Pingtung University of Science and Technology, Pingtung 912301, Taiwan[119,116,46,117,100,101,46,116,115,117,112,110,46,108,105,97,109,64,117,105,108,104,99]
Tagged With
Related Posts

Aquafeeds
Development, testing of microencapsulated Schizochytrium feeds for bivalve broodstock
Comparing performance of O. edulis broodstock fed microencapsulated Schizochytrium feed and live algae diets shows potential for bivalve hatcheries.

Aquafeeds
Fortifying shellfish with novel microencapsulated feeds could help address human nutrient deficiencies
Evaluating a novel microencapsulated feed supplied during the depuration stage to fortify oysters with micronutrients beneficial to human health.

Innovation & Investment
In Australia, aquaculture is looking up – and ahead
Pathogen-resistant crawfish and microencapsulated aquafeed supplements are two examples of how Australia’s aquaculture industry is always innovating.
Innovation & Investment
Early-stage diet innovator secures Aqua-Spark investment
The Netherlands-based investment fund is backing a proprietary micro-encapsulation technology that aims to replace live-feed needs for early-stage fish and shrimp.



