Results showed that taurine supplementation significantly promoted shrimp postlarvae survival rate, from 61.11 to 76.67 percent, in low-salinity waters and also increased shrimp body length

Although low-salinity shrimp culture technology has been successfully developed and rapidly disseminated, shrimp aquaculture in low-salinity water systems is reportedly still challenged by a lower yield per unit area and other issues. These are closely related to osmotic stress caused by low-salinity conditions; therefore, the osmoregulatory mechanism in shrimp under low-salinity stress needs to be better elucidated. Then, nutritional modulatory methods can be developed to enhance the osmoregulatory capacity and yield of shrimp freshwater culture.
The Pacific white shrimp (Litopenaeus vannamei) is a typical euryhaline (able to adapt to a wide range of salinities) crustacean and exhibits a strong ability to adapt to water environments of varying salinities via osmoregulation, which has facilitated its widespread geographical distribution. In seawater, euryhaline crustaceans act as osmoconformers (organisms that allow their body fluids to match the osmotic pressure of the surrounding water), with the osmotic pressure of their hemolymph directly reflecting that of the external environment. If water salinity drops below 26 ppt, this shrimp will activate various hyper-osmoregulatory mechanisms, which are reliant on a suite of ion transport carriers and enzymes.
Early studies in Pacific white shrimp reported the optimal taurine level to be 0.168 percent of the dry diet when salinity was at 29–30 ppt], but other authors reported it was 0.437–0.579 percent and 0.57–0.60 percent when salinity was at 5.5–6.0 ppt. Therefore, it is understandable that shrimp require substantial energy and osmolytes to adapt to osmotic changes under low-salinity conditions, thus significantly altering their nutritional demands. Further research is required on the osmoregulatory function of taurine under low-salinity conditions in shrimp at different growth periods. Current taurine research mainly focuses on shrimp juveniles and adults, with little information revealed on osmoregulatory regulation during the early developmental stages, including in shrimp postlarvae, which urgently requires further in-depth investigation.
This article – summarized from the original publication (Wang, H. et al. 2025. Taurine Supplementation Enhances the Resistance of Litopenaeus vannamei Postlarvae to Low-Salinity Stress. Biology 2025, 14(8), 1082) – reports on a study to explore taurine’s functions in shrimp postlarvae under low-salinity stress.
Study setup
This research was conducted at Huazhong Agricultural University (Wuhan University, China). Healthy L. vannamei shrimp postlarvae (PL) from the same breeding batch (salinity of 18 ppt) with uniform size were cultured in indoor experimental tanks with water of different salinities and divided into three groups: control group (C, 18 ppt), low-salinity group (L, salinity gradually reduced from 18 ppt down to 4 ppt at a rate of 2 ppt per day), and low-salinity + taurine group (T, similar salinity to L group).
A commercial shrimp diet (Evergreen Feed, Zhanjiang, China) was used as the basal diet to feed shrimp PLs in the C and L groups. The experimental diet in the T group was formulated by dissolving taurine in water and then spraying this on the basal feed pellets (0.3 percent of dry feed weight). Each treatment consisted of three replicates with 300 PLs in each experimental aquarium. Water temperature, dissolved oxygen and pH were maintained within ranges of 29 ± 1 degrees-C, 7.1 ± 0.4 mg/L and 7.4 ± 0.6, respectively. At the end of the one-week feeding trial, the shrimp PLs were collected to calculate the survival rate and body length. Some PL samples were fixed and stored for subsequent analysis.
For detailed information on the experimental design, animal husbandry and diet preparation; and animal samples and data collection and analyses, refer to the original publication.
Results and discussion
L. vannamei can live and be commercially cultured in both brackish and freshwater due to its ability to adapt to different salinity levels. In shrimp aquaculture, rainstorms and evaporation lead to changes in pond salinity that often impact shrimp performance. The shrimp’s tolerance to salinity depends on various factors like their growth stage and how quickly they are exposed to changes. While many studies have focused on adult shrimp, little is known about their early stages. This study looked at shrimp PLs under low-salinity stress and after taurine supplementation, assessing survival, growth, histology, enzyme activity and other responses.
Results show that dietary taurine can significantly improve PL survival and help repair tissue damage under low-salinity stress. Salinity is one of the most important factors that affect the physiology as well as growth of cultured shrimp. Low-salinity exposure occurring through gradual but rapid change can significantly affect the saline tolerance of shrimp juveniles and adults. In our study, in order to simulate an extremely saline environment, the water salinity for the experimental low-salinity stress (L) shrimp group was lowered from 18 to 4 ppt in a period of seven days.
As shown in Fig. 1, the survival rate of shrimp PLs in the L group dropped to 61.11 percent, compared to 92.67 percent in the control group, showing that the animals struggle with rapid salinity changes. Previous studies found that shrimp exposed to a sudden salinity drop from 30 to 0.3 ppt had a survival rate of only 11.91 percent, while those adapted more gradually had a survival rate of 86.67 percent, which was still lower than the control group in that study.
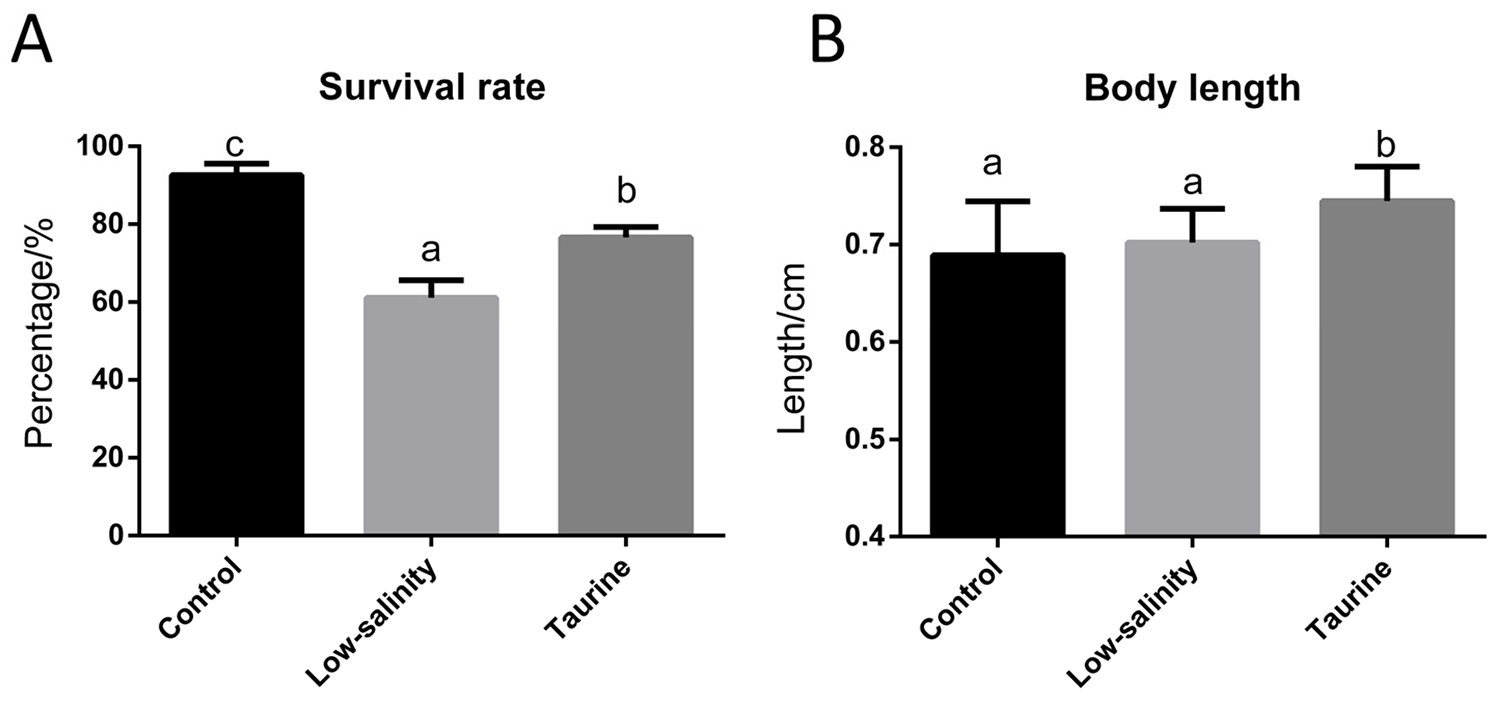
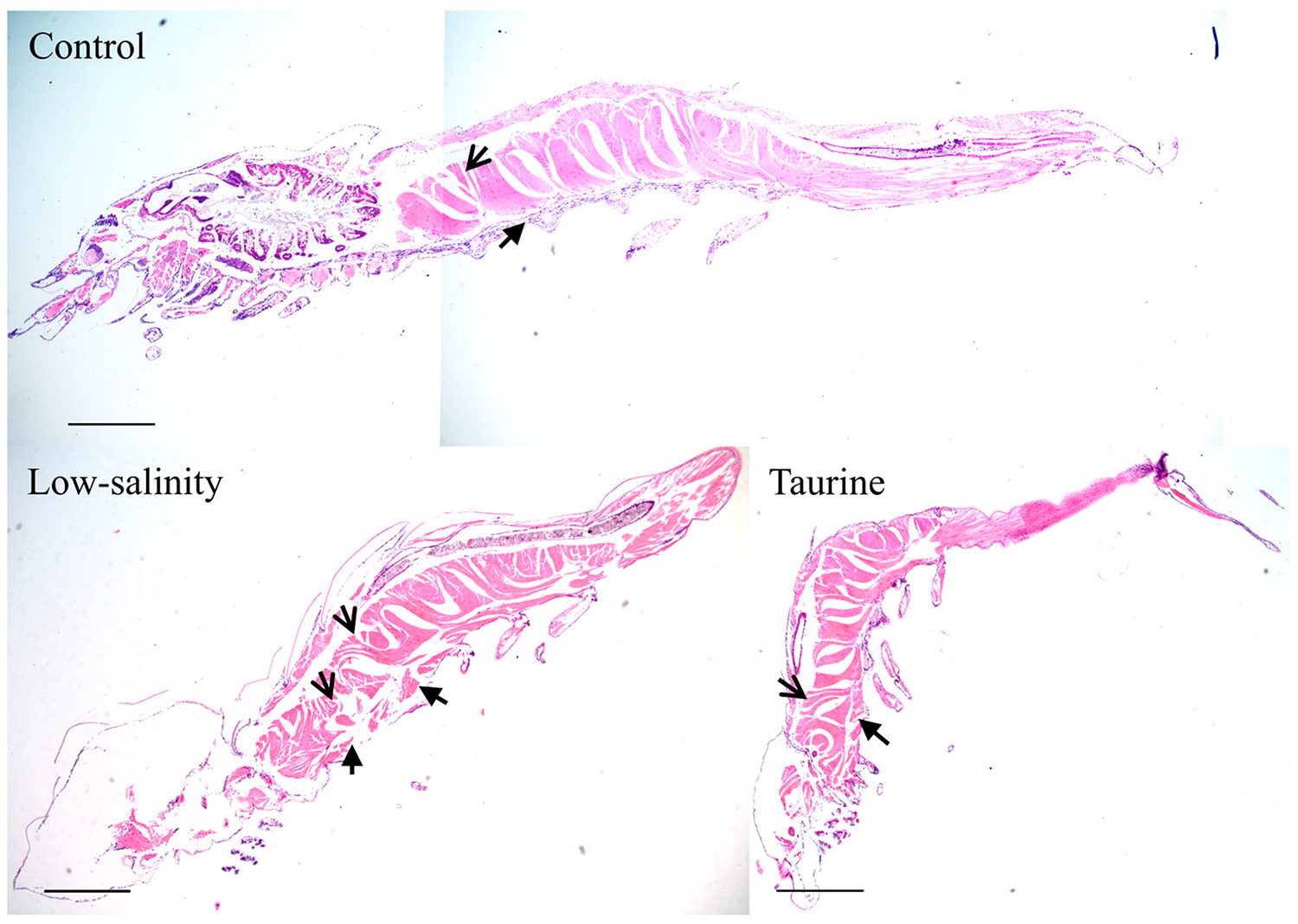
To investigate the improvement of shrimp PL performance in low-salinity conditions, taurine was added to their diet. Taurine is known to improve the activity of certain enzymes, improving animal growth and feed efficiency. Various studies have reported that taurine supplementation can help shrimp grow better, especially in low-salinity water. In this study, taurine significantly improved the survival rate of shrimp PLs under stress and led to better body growth. Additionally, it helped restore muscle fibers and organ structure compared to the low-salinity group. Overall, taurine appears to reduce tissue damage from exposure to very low salinities, and also to enhance the growth of the animals. Histological examinations in our study revealed significant differences, with the L group displaying poor tissue organization and some structural tissue damage compared to the intact tissue morphology observed for the C group.
Taurine plays a key role in helping shrimp maintain their ionic balance in low-salinity environments by regulating the enzyme Na+/K+-ATPase (NKA). This important enzyme, known as the sodium-potassium pump and present in the membranes of all animal cells, performs several critical functions in cell physiology. When shrimp experience low salinity stress, we observed that NKA protein expression and activity increased, indicating that the shrimp are adjusting to changes in their environment. This aligns with previous research showing changes in NKAα subunit mRNA levels in shrimp after they are moved to lower salinity water. The imbalanced ions in the surrounding medium also boost NKA pump activity, helping to balance osmotic pressure.
Additionally, NKA activity and expression are influenced by nutrients, and researchers have reported that a diet high in highly unsaturated fatty acids (HUFAs) reduced NKA activity in shrimp after salinity exposure. Also, that taurine, when included in shrimp diets, can counter low-salinity stress and improve growth much more than potassium supplementation. Furthermore, taurine supplementation lowered NKA activity under low-salinity stress to levels similar to those in freshwater, suggesting it efficiently helps with osmoregulation. Overall, taurine appears to aid shrimp in adjusting to low-salinity conditions, which is important for their survival and growth when cultured under these conditions.
In the study, transcriptomic analysis (Transcriptomics is the comprehensive study of the transcriptome – a collection of all the gene readouts present in a cell – which includes all ribonucleic acid, RNA, molecules found within an organism) was conducted to understand how shrimp adapt to low-salinity stress, and results revealed that 737 genes were affected, with 454 genes upregulated (turned on) and 283 genes downregulated (turned off). In shrimp exposed to low salinity, certain biological activities – such as hormone-related functions and collagen metabolism – increased, suggesting a response to osmotic regulation. Results also showed that low salinity inhibited various physiological pathways promoting cell growth and oxygen transport, leading to lower survival rates in exposed shrimp.
We also observed that when dietary taurine was added, it affected gene expression significantly, with 497 genes up-regulated and 437 down-regulated. Results also showed that taurine reduced the expression of genes involved in hormone activity and receptor signaling, indicating its role as an osmoregulator and antioxidant. Taurine also appeared to lessen shrimp’s sensitivity to environmental changes, promoting their survival. Additionally, taurine encouraged controlled cell proliferation and impacted Wnt signaling pathways – ancient pathways that regulate crucial aspects of cell fate determination, cell migration, and neural patterning and organogenesis during embryonic development – which may help repair shrimp’s gill or intestinal tissues.
Results also indicated that dietary taurine enhanced osmoregulatory protein activity and activated various metabolic pathways related to carbohydrates, amino acids and vitamins, helping the shrimp adapt to low-salinity environments. Furthermore, while steroid hormone production increased under low salinity, taurine seemed to lower this production, supporting its stress-relief properties.
Overall, taurine supplementation resulted in 529 differentially expressed genes when comparing low-salinity shrimp PLs with and without dietary taurine supplementation, showing a reduction in gene changes linked to stress, and demonstrating taurine’s therapeutic benefits for mitigating low-salinity stress in L. vannamei PLs. Additionally, taurine positively influenced developmental and neurogenic pathways, while suppressing indicators of cell death, potentially preserving cellular health in shrimp under low-salinity stress conditions.
Perspectives
In this study, low-salinity stress significantly restricted L. vannamei PL survival and caused histological changes, induced NKA overactivation, and differentially regulated gene expression. Taurine can serve as an osmolyte to effectively mitigate low-salinity stress-induced damage while simultaneously promoting epithelial cell proliferation in shrimp PLs. However, the present study was still limited by its short duration and lack of mechanistic validation. In future research, dose–response studies, longitudinal trials and proteomic validation of key targets are suggested to fully confirm the relevant regulatory mechanism.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Huaichi Wang
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
-
Xinyue Du
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
-
Jiahong Zou
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
-
Mengya Wang
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
-
Yan Lei
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
-
Bin Zhang
China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs, Guangxi Key Laboratory of Aquatic Genetic Breeding and Healthy Aquaculture, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Yongzhen Zhao
China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs, Guangxi Key Laboratory of Aquatic Genetic Breeding and Healthy Aquaculture, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Linyuan Jiang
China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs, Guangxi Key Laboratory of Aquatic Genetic Breeding and Healthy Aquaculture, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Xiaohan Chen
China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs, Guangxi Key Laboratory of Aquatic Genetic Breeding and Healthy Aquaculture, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Qingchao Wang
Corresponding author
Key Laboratory of Aquacultural Facility Engineering (Ministry of Agriculture and Rural Affairs), College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China[110,99,46,117,100,101,46,117,97,122,104,46,108,105,97,109,64,103,110,97,119,99,113]
Related Posts

Health & Welfare
Biofloc potential to alter virulence of V. parahaemolyticus strain on L. vannamei postlarvae
A biofloc environment has the potential to alter the virulence of the AHPND-causing V. parahaemolyticus strain on Pacific white shrimp postlarvae.

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Health & Welfare
Development, implementation of shrimp health programs require integrated effort
Shrimp health management focuses on disease prevention through good nutrition, sound pond management, and stress reduction rather than disease treatment.

Health & Welfare
Evaluating digestibility and performance of insect meals in diets for Pacific white shrimp
An assessment of seven insect meals demonstrates their high potential as protein sources to replace fishmeal in diets for L. vannamei.


![Ad for [BSP]](https://www.globalseafood.org/wp-content/uploads/2025/07/BSP_B2B_2025_1050x125.jpg)
