Successful BFT-based aquaculture systems favor the growth and dynamics of specific microbial communities

Bacterioplankton communities and their biological functionalities are critical components of biofloc technology (BFT) aquaculture systems. The main reason to characterize these complex microbial communities is to develop a method that can maintain a stable microbial community, supporting optimal shrimp health and water quality, as variations in microbial communities are thought to be closely associated with disease outbreaks in aquaculture systems.
Given that the microbial community determines water quality and the overall functioning of BFTs, several studies have been conducted to describe the microbiota composition of various components of the BFT system. For example, researchers investigated bacterial community composition in the intestine of the Pacific blue shrimp (Litopenaeus stylirostris) and rearing water of both clear seawater and BFT systems and observed that bacterial communities of both systems varied considerably. Subsequently, other researchers reported that carbon sources and the C/N ratio were demonstrated to influence the bacterial community composition of BFT systems. And other studies showed that the gut microbiota composition of Pacific white shrimp (L. vannamei) changes dramatically during various growth stages, and that gut microbiota share a strong resemblance with bacterial communities of large-sized bioflocs.
Overall, several studies have demonstrated variations in shrimp intestinal microbiota during various growth stages are available in the literature. However, studies delineating bacterioplankton community dynamics in rearing water at various culture stages, and its association with prevailing environmental variables in a BFT system, are rather scarce.
This article – summarized from the original publication [Cho, J-C. 2022. Exploring bacterioplankton communities and their temporal dynamics in the rearing water of a biofloc-based shrimp (Litopenaeus vannamei) aquaculture system. Front. Microbiol. 13:995699] – reports on a study to culture the shrimp L. vannamei in a greenhouse-enclosed, BFT production system, and investigate how the bacterioplankton community of the rearing water changed with shrimp growth to explore the intrinsic association between culture stages, environmental variables, and microbiota composition of rearing water.
Dynamics of bacterial communities in Pacific white shrimp larvae
Study setup
This study was conducted at a greenhouse-enclosed biofloc aquaculture system for shrimp production at the West Sea Mariculture Research Center, National Fisheries Research and Development Institute, Taean, South Korea. The prototype system had two, 300-square-meter, 0.8-meter-deep raceways enclosed in a 1,000-square-meter greenhouse structure with double-layered translucent sheets for heat insulation. The two raceways (Tank-1 and Tank-2) operated as BFT systems and rearing water was not exchanged during the trial.
Pathogen-free L. vannamei postlarvae (PLs) were procured from a commercial supplier (SyAqua Sia. Co., Ltd.) in Thailand). The PLs were nursed 16 days and then a total of 120,000 nursery-cultured juveniles (initial body weight 0.038 grams) were stocked in each raceway tank at a stocking density of 320 individuals per cubic meter and reared for 152 days. Rearing water samples were collected on a weekly basis for five months and water quality variables such as physicochemical parameters and inorganic nutrients were monitored. In parallel, sequencing of the 16S rRNA gene was employed to investigate the temporal patterns of rearing-water microbiota.
For detailed information on the experimental design, rearing system, animal husbandry, and collection of samples; bacterial diversity assessment; and statistical analyses, refer to the original publication.
Results and discussion
Successful BFT aquaculture systems need definite microbial communities that can improve water quality, productivity, and biosecurity. There is now mounting evidence that the rearing water microbiota in shrimp aquaculture systems is closely associated with shrimp intestinal microbiota, and rearing water microbiota has also been linked to shrimp disease occurrence. However, studies remain inconclusive about the bacterioplankton community composition of rearing water and how it changes with shrimp growth. In this present study, we aimed to understand the changes in physicochemical parameters and bacterioplankton community composition of rearing water during various growth stages of L. vannamei in a BFT-based aquaculture system.
Our BFT system yielded an average of 3.6 kg per cubic meter of L. vannamei shrimp, within the 3 to 6 kg per cubic meter yield reported for these BFT systems (Figure 1). This yield is also substantially higher than the 0.2–0.3 kg per cubic meter recovered from traditional flow-through culturing systems. Similarly, survival rates (89 and 74 percent in Tanks 1 and 2, respectively) in our systems are within the range reported from other BFT and traditional flow-through systems. The lower survival rate in Tank 2 might be explained by shrimp densities, which were higher than super-intensive densities (>300 individuals per cubic meter) previously reported in the literature. Feed conversion ratios (FCR) were between 1.2 and 1.3, which are similar to those previously reported in BFT systems.
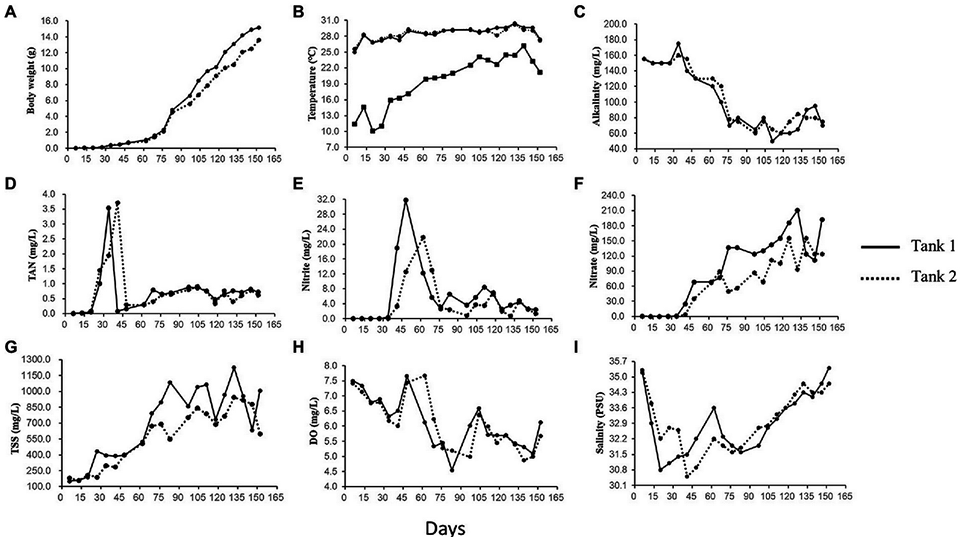
Microbial communities in the rearing water in a BFT system are involved in several crucial functions including the formation of bioflocs, maintenance of adequate water quality and shrimp health, and other biogeochemical events. Moreover, several studies investigating the microbiome composition of aquaculture systems have identified a tight link between the microbiomes of shrimp intestines and rearing water. The speculated association was explained by the constant flow of rearing water through the digestive tract of growing shrimp, which thus influences the composition of gut microflora.
In this study, we first demonstrated that the bacterioplankton community in rearing water is highly dynamic. A clear change in overall bacterioplankton community composition along the culture growth was observed. The bacterioplankton community exhibited two major shifts during the entire course of the study (Fig. 2). The first shift likely occurred between TAS4 and TAS5 (during 48–69 culture days), to which an abruptly increased abundance of Actinobacteria might have contributed majorly. Similarly, the second shift began between TAS7 and TAS8 (during 104–118 culture days), which may be partially attributed to the increased proportion of Chloroflexi and TM7 (a candidate phylum, also called Saccharibacteria). Similar patterns in the bacterial community of rearing water, along with intestine and sediment, at different culture stages were previously observed in a shrimp aquamimicry system.
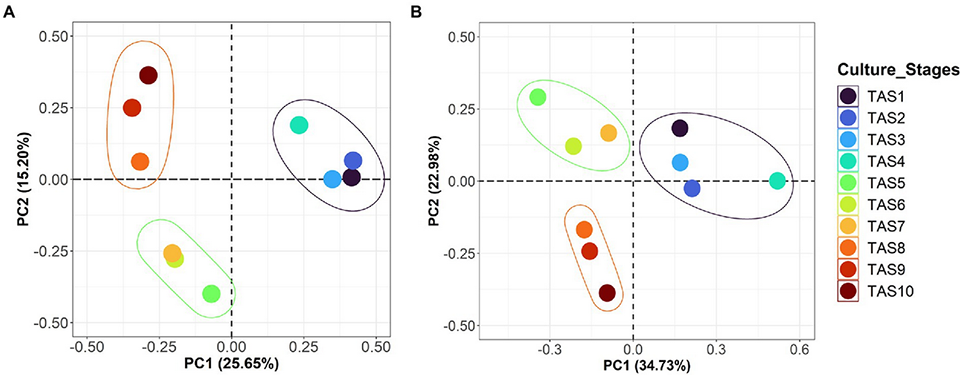
Further analysis revealed a complex microbial community in rearing water, comprising several bacterioplankton phyla such as Proteobacteria, Actinobacteria, Bacteroidetes and Planctomycetes. Proteobacteria was the most dominant phylum throughout the culture stages, which is consistent with several earlier studies. Similarly, Bacteroidetes constituted the second most abundant phylum but showed a wide range of relative abundance, ranging from 2.5 to 63 percent. This phylum contains some of the dominant bacteria in algal blooms and is known for degrading macromolecules in aquatic habitats.
The abundance of some dominant bacterioplankton phyla exhibited an inconsistent pattern during the culture stages. For instance, Cyanobacteria dominated the community in the beginning stages, but then diminished in the later culture stages. One possible explanation for such an unusual pattern is that most Cyanobacteria are free-floating photosynthetic autotrophs, and the dense turbidity of bioflocs in rearing water might have hampered their photosynthetic efficiency. In contrast to Cyanobacteria, the phylum Actinobacteria showed an entirely opposite trend by totally disappearing in the early culture stages. At the class level, rearing water microbiota was predominantly characterized by Alphaproteobacteria, Gammaproteobacteria and Bacteroidetes. The higher occurrence of these bacterioplankton groups in a BFT system is not surprising considering their principal characteristics, including the requirement of organic material and nitrogen for growth.
The rearing water of aquaculture systems has often been shown to contain potentially pathogenic bacterial genera such as Vibrio, Aeromonas, Photobacterium, Pseudomonas, Candidatus Bacilloplasma and Flavobacterium. Among these genera, the most notable pathogens are Vibrio spp. and thus quantifying their presence was prioritized in the present study. The existence of Vibrio spp. was limited (a minor abundance) in our investigation, which is consistent with earlier similar studies conducted in BFT aquaculture systems. The observed low density of Vibrio spp. might be due to the water quality and/or higher prevalence of probiotic bacterioplankton taxa that could have restricted their proliferation. Generally, the dominant bacterioplankton members of BFT systems (e.g., Proteobacteria and Bacteroidetes) compete for food and niche space, limiting the prevalence of pathogens, including Vibrio spp.
Interestingly, we observed a culture stage-specific fluctuation in the abundance of those bacterioplankton groups that could be vital for the effective performance of a BFT system. For instance, Actinobacteria, identified as a biomarker taxon during both the middle and late stages of culture, has been frequently described as playing several crucial roles in aquaculture. Actinobacteria are recommended as promising candidates for use as probiotics in aquaculture due to several characteristics, such as their ability to degrade macromolecules (e.g., protein and starch) and combat aquaculture pathogens. Previous studies have shown that feed supplemented with members of Actinobacteria (e.g., genus Microbacterium), could provide health benefits to shrimp by promoting growth and limiting the occurrence of diseases. Additionally, Actinobacteria are considered essential for maintaining gut homeostasis.
Perspectives
We investigated the physicochemical properties and bacterioplankton community of rearing water in a BFT-based L. vannamei aquaculture system. Our study uncovered the plausible factors shaping the structural composition of the rearing water microbiome, which may help in maintaining rearing water quality and mitigating the occurrence of bacterial diseases in aquaculture systems.
Based on our findings, it is reasonable to postulate that shrimp health and production in a BFT-based aquaculture system are largely reliant on rearing water microbiota, besides the shrimp’s own intestinal microbiota. In future studies, it would certainly be interesting to illustrate microbial functional processes in aquaculture systems using shotgun metagenomic and transcriptomic approaches.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Jang-Cheon Cho
Corresponding author
Department of Biological Sciences and Bioengineering, Inha University, Incheon, South Korea[114,107,46,99,97,46,97,104,110,105,64,99,106,111,104,99]
Tagged With
Related Posts

Intelligence
Biofloc technology production promising in temperate zones
A study was conducted to assess the feasibility to grow Channel catfish (Ictalurus punctatus) in an outdoor biofloc system during winter in a temperate zone. High biomasses of market-size channel catfish were successfully maintained through the winter with high survival and in good condition in both treatments.
Health & Welfare
Do current shrimp practices favor EMS?
After refilling ponds, surviving microorganisms – including Vibrio parahaemolyticus, which causes EMS in shrimp – may benefit from the availability of nutrients in sediment and water and lack of competing microorganisms.
Health & Welfare
Field diagnostic tools for pathogenic vibrios
Since Vibrio samples often degrade during transport, the authors analyzed nucleic acids (FTA) cards as a simplified, shelf-stable collection method for the preservation of genetic material from water samples.

Health & Welfare
Partial harvest-biofloc system promising for Pacific white shrimp
In a study in Indonesia, increases in efficiency in power use resulted from the combined application of biofloc technology and partial harvest.



