An optimal stocking density for intensive culture in biofloc systems balances high shrimp yield with manageable water quality and good growth performance

Biofloc technology (BFT) is a promising sustainable aquaculture production system that utilizes microbial communities to convert toxic nitrogenous wastes into microbial protein while simultaneously improving water quality and providing supplemental nutrition. This approach offers significant advantages including dramatically reduced water exchange rate (<10 percent), improved nutrient utilization efficiency, and enhanced biosecurity through competitive exclusion of pathogens.
Despite these benefits, critical knowledge gaps remain regarding the optimization of stocking density in BFT systems – a key parameter that directly affects productivity, water quality, and economic viability. Previous studies have been limited by small-scale experimental designs that do not reflect commercial conditions, short trial durations insufficient to capture full grow-out cycles, narrow density gradients (typically ≤ 600 ind/m3), and incomplete assessments of nitrogen dynamics and their management implications. These limitations have hindered the development of practical guidelines for commercial-scale BFT implementation, particularly regarding the density-dependent trade-offs between production yield and harmful nitrogen control.
This article – summarized from the original publication (Xu, W. et al. 2025. Effect of Stocking Density on Water Quality, Harmful Nitrogen Control, and Production Performance of Penaeus vannamei in Biofloc-Based Systems with Limited Water Exchange. Fishes 2025, 10(7), 326) – reports on a study that This study addresses these knowledge gaps through a comprehensive, production-scale evaluation of four stocking densities (200, 400, 600, and 800 ind/m3) over a complete 56-day grow-out cycle.
Study setup
This research was conducted in 12 biofloc systems – concrete tanks, 6×6×1 meters, 30-cubic-meter water volume with water injectors and recirculating pumps – with identical physical conditions, housed in a temperature-controlled greenhouse with semi-shaded plastic film cover. The water source was natural seawater that underwent sand filtration and chlorination prior to use. To accelerate biofloc development, each system was inoculated with mature biofloc water from a shared source of the previous production cycle.
The trial used a completely randomized design to evaluate four stocking density treatments (200, 400, 600, and 800 individuals per cubic meter, or ind/m3), denoted as D200, D400, D600, and D800, respectively). Each treatment was replicated in three independent biofloc-based systems, resulting in a total of twelve experimental units.
Healthy P. vannamei juveniles were sourced from a single nursery pond and individuals with a mean weight of 0.36 ± 0.14 grams (mean ± S.D.) were selected and stocked into the trial systems according to the four stocking densities. Density-specific biomass was precisely weighed and distributed to achieve target loadings (2.16, 4.32, 6.48 and 8.64 kg/tank for D200, D400, D600 and D800, respectively), with <2 percent variation from targets based on weight measurement.
The 56-day culture period encompassed the critical grow-out phase from juvenile to marketable size. Water temperature was maintained at 28.0 ± 2.0 degrees-C using greenhouse climate control systems, with continuous aeration ensuring dissolved oxygen concentration above 5.0 mg per liter throughout the trial period.
For detailed information on the experimental design, BFT system, animal husbandry, dan data collection and analyses, refer to the original publication.
Results and discussion
A significant density-dependent pattern was observed in management inputs. Water exchange rates increased proportionally with stocking density, ranging from 0.6 percent per day at 200 ind/m3 (D200) to 7.9 percent per day at 800 ind/m3 (D800). Similarly, molasses and sodium carbonate usage showed density-dependent increases, with the highest inputs recorded in D800 (41.8 kg per tank and 57.3 kg per tank, respectively). During the trial, as the stocking density of shrimp was increased from 200 to 800 ind/m3, the required water exchange rate, molasses usage, and sodium carbonate usage were found to increase by 1217, 160 and 321 percent, respectively.
The dissolved oxygen (DO), pH, total ammonia nitrogen (TAN), and nitrite nitrogen (NO2—N) of the system were significantly affected by stocking density. DO and pH levels declined from 6.7 mg/L to 5.1 mg per liter and 7.6 to 7.3, respectively, with a density increase from 200 to 800 ind/m3, while TAN and nitrite nitrogen concentrations increased from 0.23 mg per liter to 0.96 mg per liter and 0.47 mg per liter to 1.68 mg per liter, respectively, with a density increase from 200 to 800 ind/m3. Notably, the threshold effects for TAN and nitrite nitrogen significantly deteriorated above 600 ind/m3. During the trial, as the stocking density of shrimp increased, DO and pH levels decreased between 24 and 4 percent, respectively, while TAN and nitrite nitrogen concentrations increased by 317 and 257 percent, respectively.
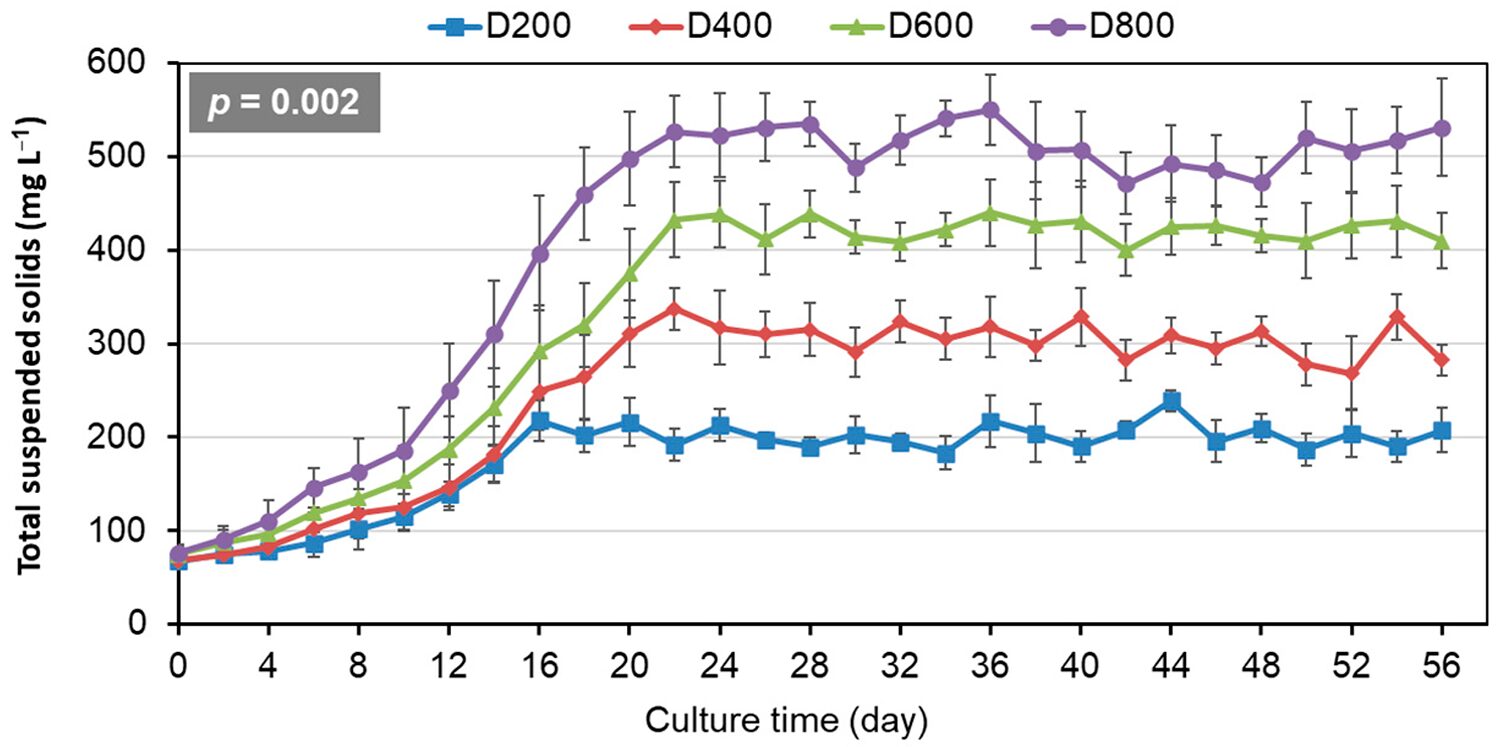
Regarding biofloc control and nitrogen dynamics, total suspended solids (TSS) showed similar increase trends initially and then stabilized across different stocking densities (Fig. 1). Significantly higher TSS were maintained as stocking density increased from 200 to 800 ind/m3. To maintain TSS around target ranges of 200, 300, 400, and 500 mg per liter for D200, D400, D600, and D800, respectively, water exchange rates were adjusted proportionally to stocking density.
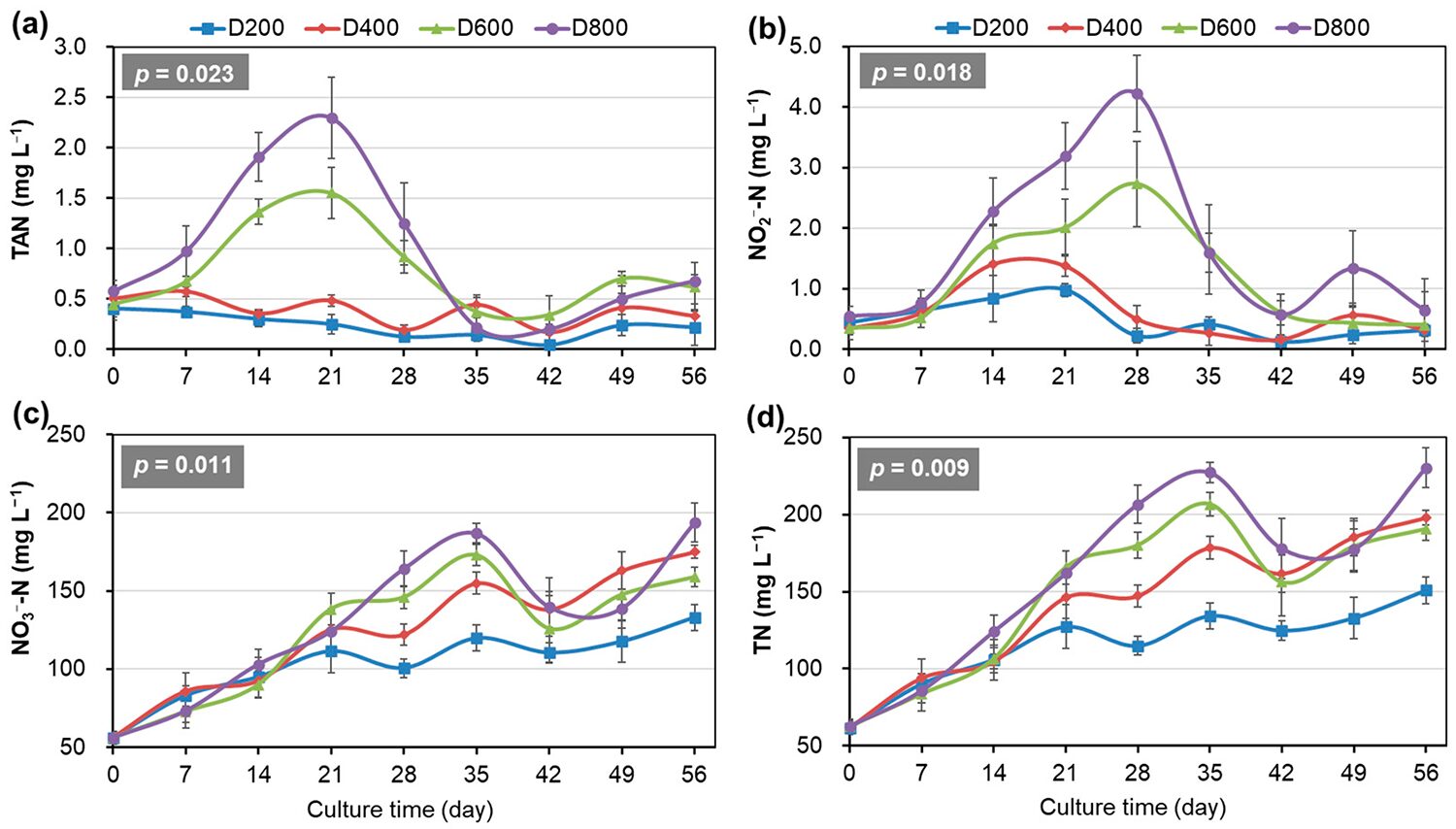
The dynamics of nitrogen compounds displayed pronounced density-dependent fluctuations (Fig. 2). The concentrations of both TAN and nitrite nitrogen increased and then decreased during the early to middle phase of the trial, respectively, and then were maintained at almost below 1.0 mg per liter until the end of the trial in all systems (Fig. 2a,b). During the peaking stage, the concentrations of TAN and nitrite nitrogen were significantly higher and lasted longer when the stocking densities of the system were higher.
Notably, in D800, TAN reached a peak of 2.3 mg per liter on week 3, and its concentration above 1.0 mg/L lasted for more than three weeks, while nitrite nitrogen peaked at 4.2 mg per liter at week 4, and its concentration above 1.0 mg per liter lasted for more than four weeks (Fig. 2a,b). The concentrations of nitrate nitrogen (NO3 –-N) and total nitrogen (TN) increased in volatility during the early and middle phases of the trial and showed irregular fluctuation later on in all systems (Fig. 2c,d). Nitrate nitrogen and TN accumulation was significantly higher when the stocking densities of the systems were higher.
Shrimp production performance varied significantly across stocking densities. While yield increased from 3.62 kg per cubic meter in D200 to 9.09 kg per cubic meter in D800, individual growth metrics declined. Harvest body weight decreased from 19.14 grams in D200 to 14.12 g in D800, while size variation increased from 14.03 percent in D200 to 23.90 percent in D800. Specific growth rates dropped from 7.10 percent per day in D200 to 6.62 percent per day in D800, and survival rates decreased from 94.6 percent in D200 to 79.8 percent in D800. The feed conversion ratio worsened with density, rising from 1.12 ± 0.03 (D200) to 1.29 ± 0.05 (D800). During the trial, as the stocking density of shrimp was increased from 200 to 800 ind/m3, harvest body weight, specific growth rate and survival rate were observed to decrease by 36, 7 and 19 percent, respectively, while size variation, yield and feed conversion rate increased by 70, 151 and 15 percent, respectively.
Significant increases in system management inputs were observed with higher stocking densities of P. vannamei in biofloc-based systems during the 56-day culture period. Water exchange rates increased to maintain target TSS levels for four stocking densities, reflecting the substantial inputs of feed and accumulation of metabolic wastes at higher densities. Similarly, molasses and sodium carbonate addition increased significantly, indicating greater microbial demand for nitrogen transformation and pH regulation. These findings align with those of other researchers, who reported similar pH instability in systems exceeding 500 ind/m3 while extending their observations to commercial-scale operations.
Critical water quality parameters exhibited significant variations across stocking densities of shrimp. DO concentrations progressively declined across the density gradient, while pH maintenance became increasingly challenging despite continuous buffering. These results corroborate previous studies regarding oxygen consumption and pH stability in biofloc systems at high density of shrimp. Notably, our improved TSS control protocol (maintained at 200-500 mg per liter through regulated water exchange) represents a significant advancement over earlier studies.
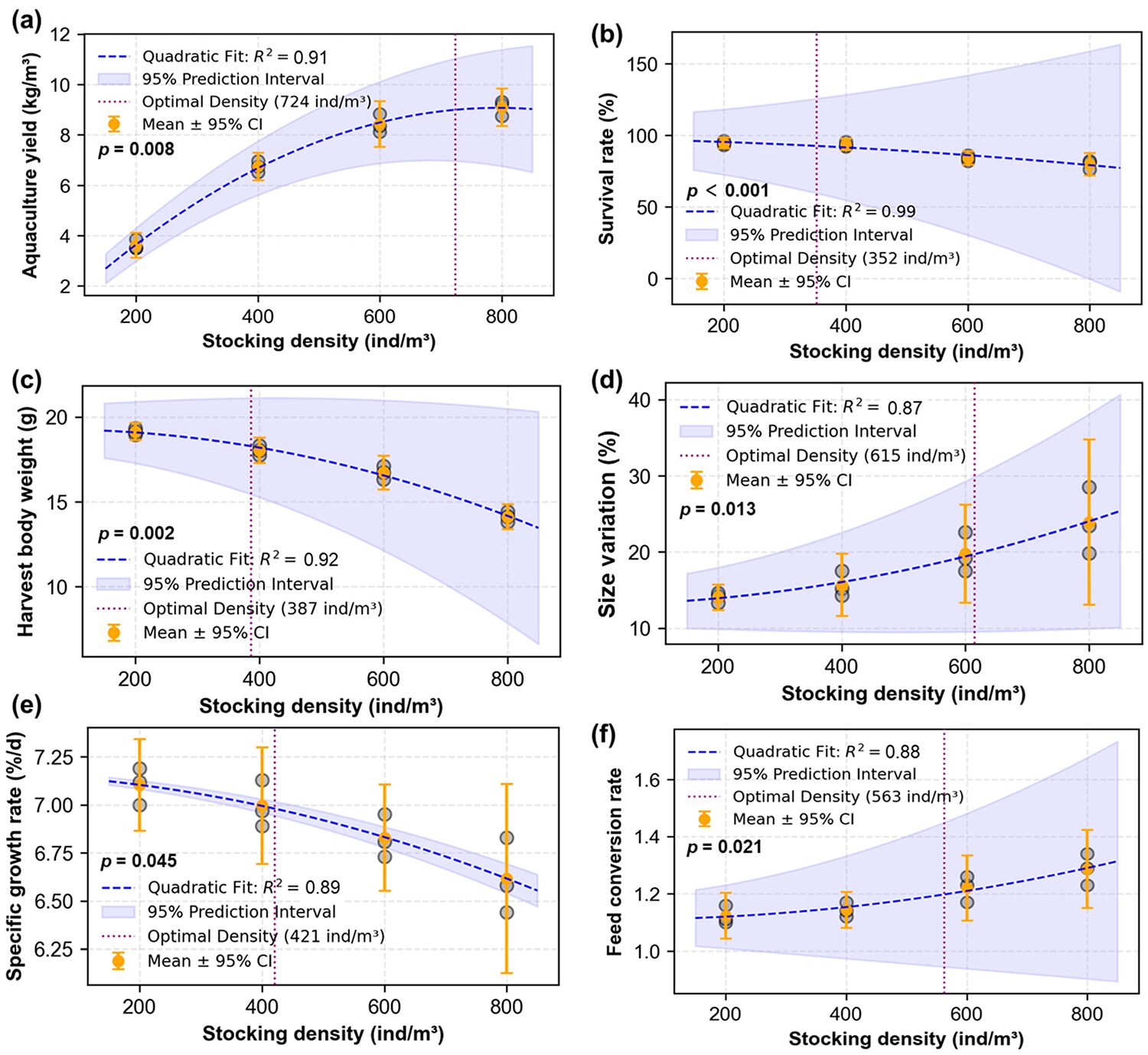
The management intensity required to maintain water quality parameters within optimal ranges increased with the stocking density of shrimp. Our data suggest that operational complexity and resource requirements escalate disproportionately beyond 600 ind/m3, supporting the practical density thresholds proposed by previous studies. The results have important implications for commercial operations, as they demonstrate that marginal production gains at higher densities must be carefully weighed against exponentially increasing management demands. These insights are particularly valuable for farmers implementing biofloc technology at production scale.
The harmful nitrogen dynamics observed in this study have important implications for commercial biofloc operations. The increase in harmful nitrogen accumulation beyond 600 ind/m3 suggests a critical threshold for system stability. Our findings indicate that without proper intervention strategies, such as optimized carbon supplementation or microbial augmentation, high-density systems may face persistent harmful nitrogen control challenges. These results demonstrate that the costs of harmful nitrogen management must be carefully considered when determining optimal stocking densities. Future research should focus on developing targeted approaches to enhance nitrogen processing efficiency in high-density biofloc systems while maintaining shrimp health and performance.
Through integrated weighting of all parameters monitored, the study determined 513 ind/m3 (prioritizing yield: 60 percent; FCR: 20 percent; and harvest weight: 20 percent) and 529 ind/m3 (yield: 60 percent; survival rate: 20 percent; and harvest weight: 20 percent) as optimal stocking densities, both within the validated range of 400-600 ind/m3 that aligns with lab-scale evidence of enhanced microbial nitrogen processing and commercial observations of stress mitigation at moderate densities. Practical recommendations include (1) adopting 400-600 ind/m3 to balance shrimp productivity and system input, and (2) implementing real-time monitoring of TAN and nitrite nitrogen to address density-dependent harmful nitrogen stress.
Perspectives
This study evaluated the effects of stocking density (200–800 ind/m3) on P. vannamei production performance in biofloc-based systems, demonstrating that higher densities significantly increased harmful nitrogen fluctuation and operational demands while compromising shrimp growth and feed efficiency. The optimal density range of 400–600 ind/ m3 balanced yield (6.74–8.43 kg/m3) with manageable water quality, providing a practical guideline for farmers to optimize production sustainability.
For commercial applications, we recommend adopting densities of 400-600 ind/m3, implementing real-time water quality monitoring, and adjusting carbon supplementation to mitigate nitrogen stress. Future research should focus on microbial community engineering to enhance nitrogen processing, long-term shrimp health impacts under density stress, and cost–benefit analyses for large-scale implementation.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Wujie Xu
Corresponding author
Key Laboratory of Aquaculture Genetic and Breeding and Healthy Aquaculture of Guangxi, Key Laboratory of Comprehensive Development and Utilization of Aquatic Germplasm Resources of China (Guangxi) and ASEAN (Co-Construction by Ministry and Province), Ministry of Agriculture and Rural Affairs, Guangxi Academy of Fishery Sciences, Nanning 530021, China[110,99,46,99,97,46,105,114,102,115,99,115,64,101,105,106,117,119,117,120]
-
Bin Zhang
Key Laboratory of Aquaculture Genetic and Breeding and Healthy Aquaculture of Guangxi, Key Laboratory of Comprehensive Development and Utilization of Aquatic Germplasm Resources of China (Guangxi) and ASEAN (Co-Construction by Ministry and Province), Ministry of Agriculture and Rural Affairs, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Yongzhen Zhao
Key Laboratory of Aquaculture Genetic and Breeding and Healthy Aquaculture of Guangxi, Key Laboratory of Comprehensive Development and Utilization of Aquatic Germplasm Resources of China (Guangxi) and ASEAN (Co-Construction by Ministry and Province), Ministry of Agriculture and Rural Affairs, Guangxi Academy of Fishery Sciences, Nanning 530021, China
-
Yucheng Cao
South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences/Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Guangzhou 510300, China
Tagged With
Related Posts

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.

Responsibility
Malaysia shrimp farm redesign successfully combines biosecurity, biofloc technology
Blue Archipelago’s re-engineering of a large shrimp farm to a modular, biosecure facility set a benchmark for future shrimp culture projects in Malaysia.

Health & Welfare
Survival of Pacific white shrimp juveniles after exposure to critical oxygen levels in biofloc culture
A study in Brazil evaluated the survival of Pacific white shrimp juveniles after exposure to critical oxygen levels in biofloc culture conditions.

Health & Welfare
Managing nitrifying bacteria in biofloc culture of Pacific white shrimp
It is possible to maintain a microbial community in artificial substrates, after a period of absence of shrimp, aeration and even water.


![Ad for [Aquademia]](https://www.globalseafood.org/wp-content/uploads/2025/07/aquademia_web2025_1050x125.gif)
