Study results showed the protein EhPTP2 is critical in the life cycle of EHP and that targeting EHP mRNA in host cells can be effective

The microsporidian Enterocytozoon hepatopenaei (EHP) infects the hepatopancreas (HP) and intestine of penaeid shrimps including farmed Pacific white shrimp (Penaeus vannamei) and black tiger shrimp (P. monodon). A severe infection in shrimp leads to retarded growth and a subsequent reduction in productivity.
Microsporidia are obligate intracellular parasites with a characteristic infectious spore that contains an invasion organelle called a polar tube. A double-layered spore wall, consisting of an outer layer (exospore) and a chitin-rich inner layer (endospore), allows the spore to withstand harsh external environments.
Upon induction or under opportune conditions, the spore extrudes its polar tube to pierce a host cell in order to initiate an intracellular life cycle in a process called germination. The polar tube serves as a conduit through which sporoplasm (contents of a spore of certain parasitic microorganisms, which is injected into and infects a host cell) is transferred into the host cell. Many stimuli have been reported to trigger spore germination as reviewed by Weiss and colleagues. The polar tube is made of different polar tube proteins, and to date, seven polar tube proteins have been identified and named PTP1 to PTP7.
Heat inactivation of EHP spores has been demonstrated to prevent spore extrusion, which consequently inhibits the infectivity of the spore, suggesting that the spore extrusion process is a prerequisite for EHP infection in shrimp. RNA interference (RNAi) is a technique that has been applied in various studies of shrimp viruses in both preventative and therapeutic settings. Previous studies support the hypothesis that the silencing of PTPs by RNAi would reduce microsporidian infection.
In this study, we hypothesized that the polar tube protein EhPTP2 is associated with spore germination. Therefore, we predicted that silencing of EhPTP2 would inhibit EHP replication, reduce EHP copy numbers, and reduce EHP transmission. This research provides a platform for further development of possible therapeutic or preventive treatments for EHP infection based on the suppression of EhPTP2.
This article – summarized from the original publication (Yuanlae, S. et al. 2024. Shrimp injection with dsRNA targeting the microsporidian EHP polar tube protein reduces internal and external parasite amplification. Sci Rep 14, 4830 (2024) – reports on a study to determine if silencing of EhPTP2 would inhibit EHP replication, reduce EHP copy numbers, and reduce EHP transmission.
Study setup
To identify proteins involved in the spore germination process, EHP spores were isolated from the hepatopancreas (HP) of EHP-infected P. vannamei. Spores were incubated and germinated, and the most abundant EHP spore proteins were selected for analysis.
We hypothesized that the presence of one of these polar tube proteins, EhPTP2, might be required for successful EHP spore extrusion. To test this hypothesis, we injected EhPTP2-specific double-stranded RNA (dsRNA) into healthy shrimp and found that it significantly diminished EHP copy numbers in infected shrimp.
For detailed information on the experimental design and procedures used, refer to the original article.
Robins McIntosh on everything you need to know about EHP and shrimp farming, part 1
Results and discussion
Our results showed that two methods – Immunofluorescence Assay (IFA; a significant technique commonly used in immunology or molecular biology for detecting the presence and distribution of specific proteins) and Immunohistochemistry (IHC; a form of immunostaining to selectively identify proteins in cells and tissue) – that detect EhPTP2 protein expression can be used to monitor EHP spore germination and localization in infected shrimp.
EHP spore detection through light microscopy is challenging due to the requirement for a high-magnification lens (100x). Targeting specific genes or proteins associated with EHP can offer more sensitive and specific detection. The polar tube protein 2 of EHP (EhPTP2) serves as a valuable target for detection due to its involvement in the polar tube extrusion process, which constitutes a critical step in the parasite’s life cycle.
Our research indicates that targeting the EhPTP2 protein can be employed to detect extruded EHP spores in isolated spore samples. This finding suggests that IFA could be beneficial for monitoring EHP spore germination.
Furthermore, beyond the detection of live spores, EhPTP2 can also be utilized as a target for detecting EHP infection in shrimp histological sections using IHC (Fig. 1). Our finding supports the use of EhPTP2 detection as a diagnostic method for EHP infection, which is consistent with previous reports of EhPTP2 detection using other technologies.
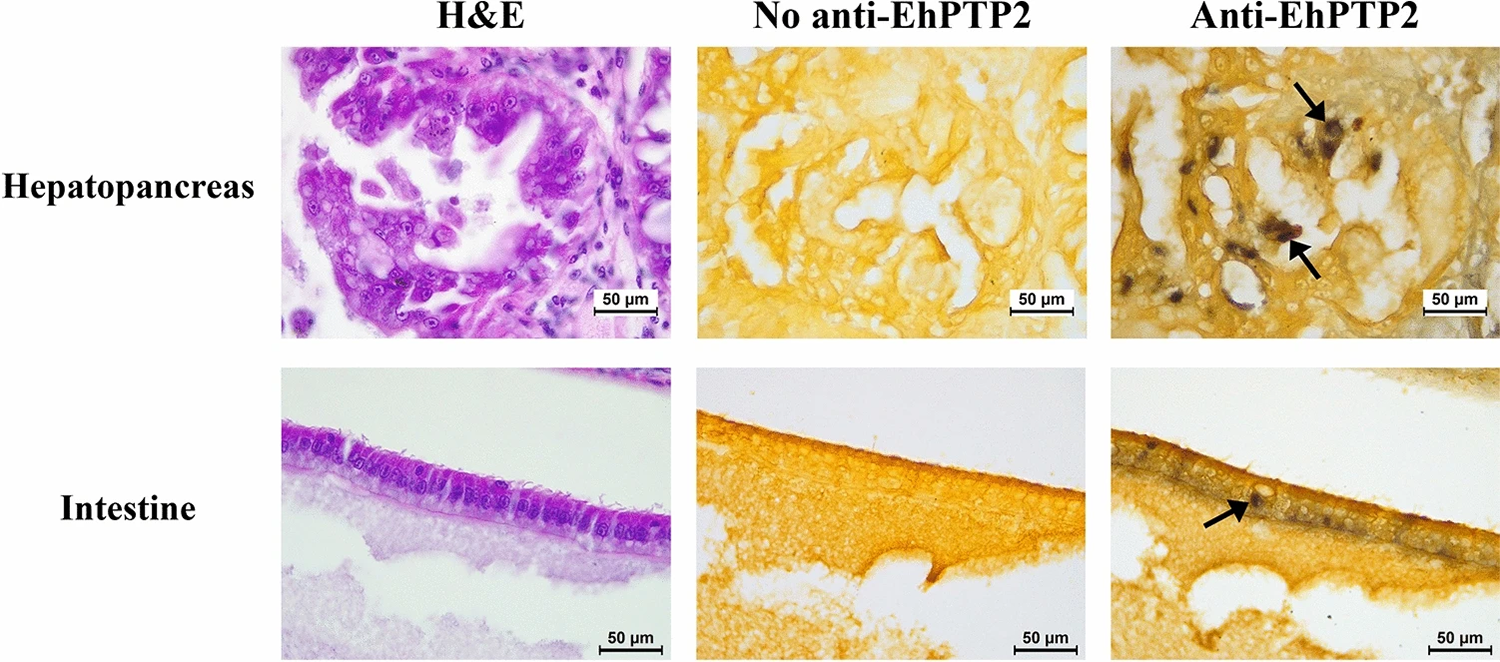
Results also showed that germination in the intestine may not lead to a successful infection. In addition to germinating in the hepatopancreas (HP) as in previous reports, a small number of germinated spores were isolated from the intestine and detected by immunohistochemistry of EhPTP2. Along the digestive tract of shrimp, there is a gastric sieve (GS) which serves as a size-exclusion filter for particles of approximately 0.2–0.7 micrometers in diameter.
Larger particles are diverted to the intestine instead of the HP. Since the average size of EHP oval spores is approximately 1 micron long, it is possible that the germinated spores detected in the intestine were derived from those that were size-excluded from the HP by the GS. While germinated spores and positive signals of EhPTP2 from immunohistochemistry were detected in the intestine, the evidence remains insufficient to conclude that the intestine is another proliferation site of EHP. Instead, we speculate that the presence of the germinated spores in the histology result was the remnant of fecal contamination rather than an active infection.
Regarding the therapeutic and preventative applications of EhPTP2 suppression, as EhPTP2 is associated with polar tube extrusion, it suggests that suppression of EhPTP2 messenger ribonucleic acid (mRNA; involved in protein synthesis in cells) would inhibit the extrusion process and result in reduced EHP infection. This was indeed the case when EhPTP2-specific double-stranded ribonucleic acid (dsRNA; associated with most viral infections, it either constitutes the viral genome in the case of dsRNA viruses or is generated in host cells during viral replication) was intramuscularly injected into previously EHP-infected shrimp (Fig. 2).
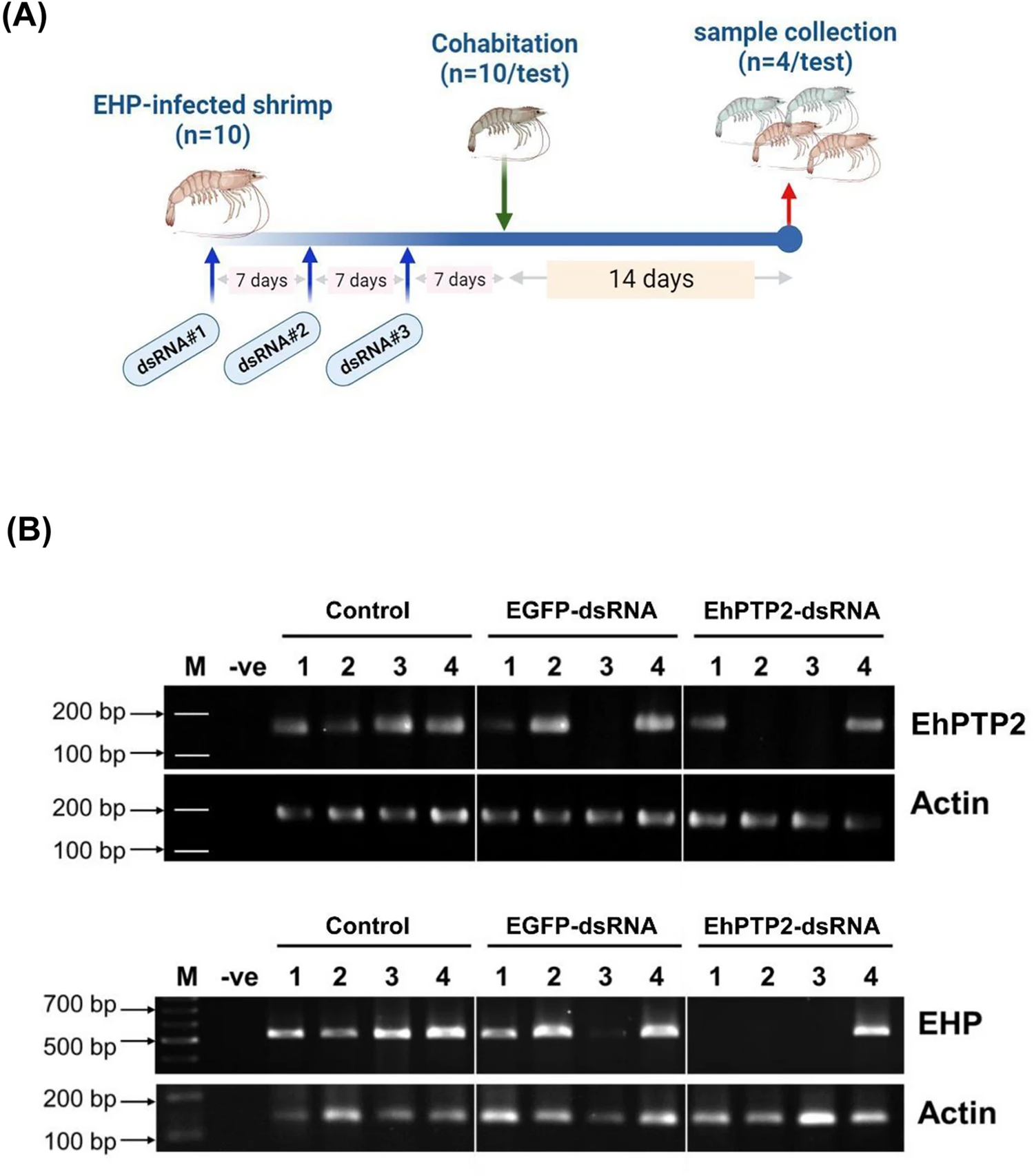
The inhibition of infection when the RNAi technique is applied preventatively is not uncommon when key virulent genes are knocked down in naïve shrimp prior to pathogen exposure. For example, in one cultured pond, high variation of EHP infection levels in the population was observed. The 25 percent of shrimp injected with dsRNA and showed EHP infection might be those infected with high levels of EHP. If the fourth dose of dsRNA-EhPTP2 can be applied, it might be able to reduce the infection. What was promising, in this study, is the fact that administration of EhPTP2-specific dsRNA could be utilized therapeutically once infection had already been established in two aspects: (1) reducing EHP loads in infected shrimp and (2) reducing further EHP spread in shrimp ponds. Only a handful of reports have documented successful therapeutic applications in pathogen-infected shrimp.
Considering that EHP infection is chronic and that the negative impact on growth is not noticeable until the EHP copy number reaches a critical threshold of 103 copies per nanogram of total hepatopancreas DNA, available data suggest that the combination of routine surveillance and therapeutic application of dsRNA against EHP polar tube protein EhPTP2 may have potential for controlling EHP.
Perspectives
The results of this study revealed that EhPTP2 plays a crucial role in the life cycle of EHP and that dsRNA targeting EHP mRNA can effectively reach the parasite developing in host cells. This approach is a model for future investigations to identify critical genes for EHP survival and spread as potential targets for preventive and therapeutic measures in shrimp.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Chanadda Kasamechotchung
Corresponding author
Department of Fisheries, Faculty of Agriculture and Natural Resources, Rajamangala University of Technology Tawan-ok, Chonburi, 20110, Thailand[104,116,46,99,97,46,111,116,116,117,109,114,64,97,107,95,97,100,100,97,110,97,104,99]
Tagged With
Related Posts

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Aquafeeds
Go beyond PCR for better aquafeed biosecurity
Authors discuss issues with using polymerase chain reaction (PCR) for pathogen detection regarding the biosecurity of formulated aquafeeds.

Health & Welfare
New management tools for EHP in penaeid shrimp
Authors examined the histological features from shrimp infected with the emerging microsporidian parasite Enterocytozoon hepatopenaei (EHP). A PCR assay method was used to detected in hepatopancreatic tissue, feces and water sampled from infected shrimp tanks, and in some samples of Artemia biomass.

Health & Welfare
Robins McIntosh on everything you need to know about EHP and shrimp farming, part 2
Shrimp farming expert Robins McIntosh details the past decade of learning about the microsporidian EHP and how to mitigate its impact.



