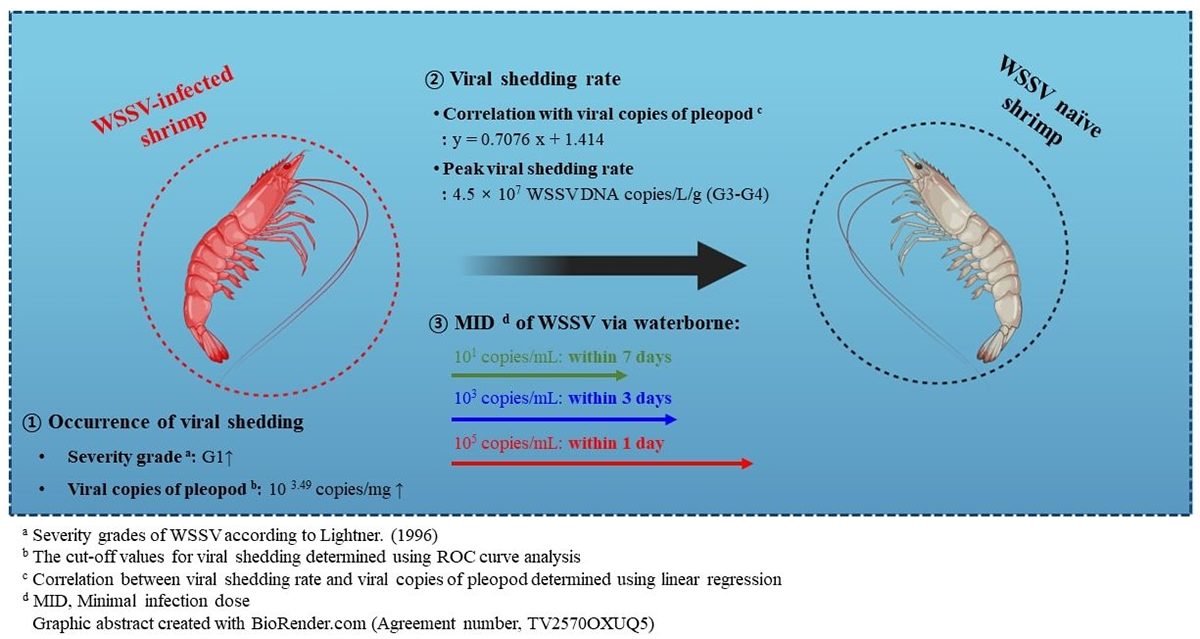
A positive correlation between disease severity grade and viral shedding rate of infected shrimp suggests WSSV waterborne transmission depends on viral load, exposure period

White Spot Syndrome Virus (WSSV), which causes White Spot Disease (WSD), is the most important crustacean pathogen. Similar to most pathogens in invertebrates, the waterborne transmission route is one of the most important horizontal transmission mechanisms for WSSV, along with cannibalism. Therefore, understanding the waterborne transmission model is crucial to preventing the outbreak and spread of diseases.
Notably, viral shedding (the expulsion and release of virus progeny following successful reproduction during a host cell infection) and minimum infective dose are important parameters for estimating and predicting viral loads in seawater, determining the exposure levels for naïve individuals, and understanding the waterborne transmission mechanism of aquatic animal diseases. Several studies have investigated the waterborne transmission of several important viruses, and although several studies have established the severity grade of WSSV and investigated the minimum infective dose via immersion challenges, no studies have investigated waterborne transmission based on the correlation between disease severity grade and viral shedding rate.
This article – summarized from the original publication (Kim, M-J. et al. 2023. Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate. Animals 2023, 13(10), 1676 – presents the results of an investigation of the correlations among clinical changes, disease severity grade, and viral copies of WSSV-infected shrimp using artificial WSSV-infected Pacific white shrimp (Litopenaeus vannamei) shrimp at different temperatures.
Study setup
Juvenile L. vannamei shrimp (2.03 ± 0.85 grams) were obtained from an aquaculture farm in Geoje, Korea, and they were confirmed to be WSSV-free using nested PCR. The shrimp were acclimated in a 250-liter aqua tank at 25 ± 0.5 degrees-C for one week and fed a commercial diet once daily. The virus used in the present study was extracted from diseased L. vannamei shrimp in Taean, Korea in 2014.
To determine the minimum infective dose of WSSV via the waterborne route, we performed immersion challenges at different administration doses and exposure periods. And we verified the minimum infective dose of WSSV and its waterborne transmission dynamics based on viral loads in shrimp and seawater via a cohabitation challenge. For detailed information on the experimental setup, animal husbandry, WSSV challenges and molecular analyses and statistical analysis, refer to the original publication.
Results and discussion
The viral shedding rate and minimum infective dose of WSSV are some of the most important factors that need to be investigated in waterborne transmission models of aquatic animal pathogens. Furthermore, because WSSV-infected shrimp exhibit various clinical changes (such as viral shedding and mortality) that vary by severity grade, understanding the correlation between viral shedding and severity grade is important for assessing the potential impact of infected shrimp on naïve individuals.
Our study investigated the correlation among severity grades, viral copies in pleopods and clinical changes (viral shedding and mortality) of infected shrimp via injection challenges at different administration doses and temperature conditions (experiments 1–2). Based on the correlation between severity grades and viral shedding rates, we verified the waterborne transmission of WSSV based on minimum infective doses and viral loads in seawater via immersion and cohabitation challenges (experiments 3–5).
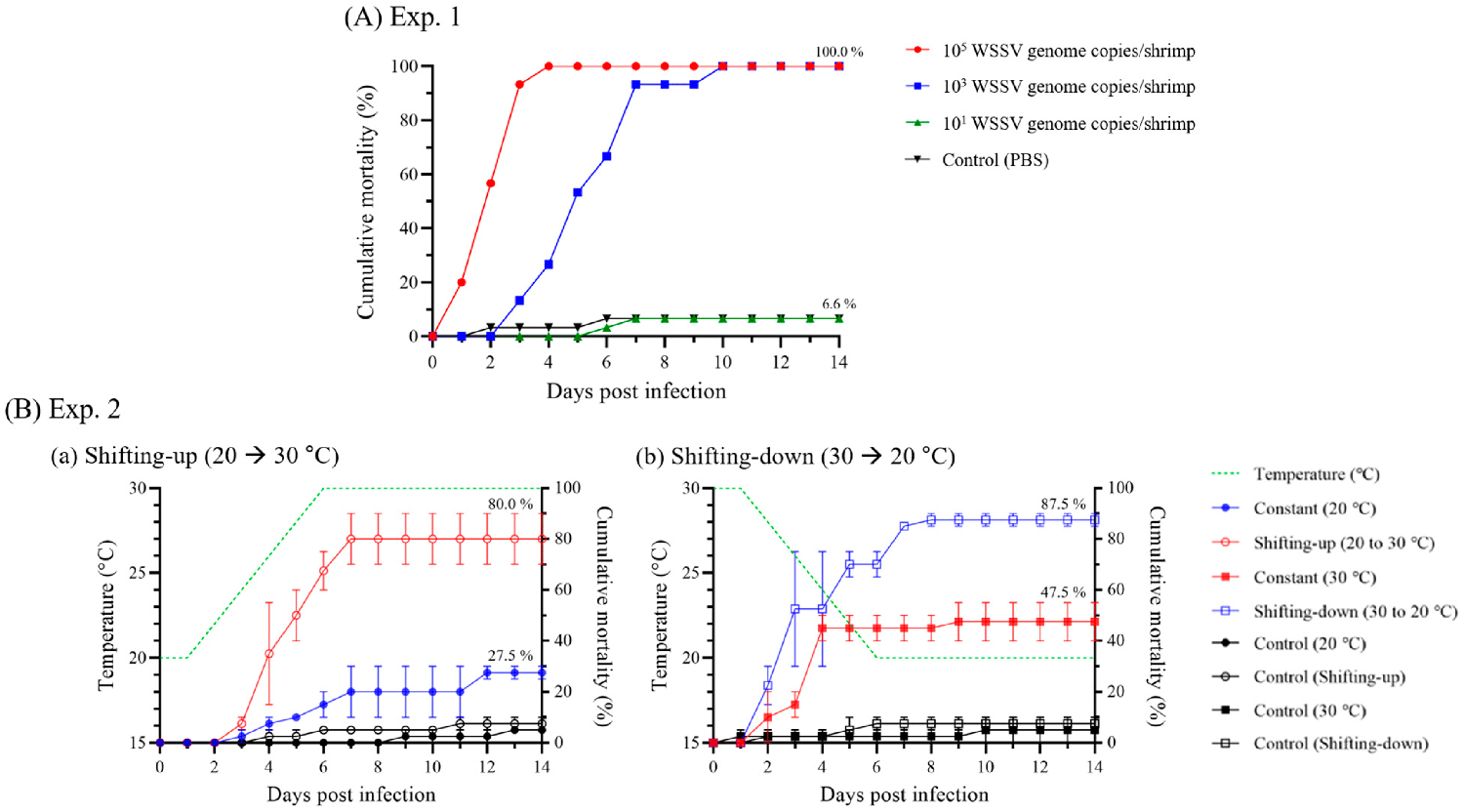
As infection and mortality inducible doses, shrimp administered 105– and 103-WSSV genome copies exhibited 100 percent cumulative mortality at four and 10 days post-infection (dpi), respectively, exhibiting significant differences depending on the initial dose administered (Fig. 2A).
Based on the results of experiment 1, experiment 2 carried out a challenge test, administering 103-WSSV genome copies doses at a water temperature range of 20 to 30 degrees-C, which is commonly reported in shrimp culture ponds. Although WSSV induced infection at 20 and 30 degrees-C water temperatures, the optimal temperature range for WSSV propagation was reported to be 23 to 28 degrees-C. As in a previous study, the cumulative mortality of WSSV-infected shrimp was significantly increased in the shifting-up (80 percent) and shifting-down (87.5 percent) groups compared to the constant (20 degrees-C; 27.5 percent) and constant (30 degrees-C; 47.5 percent) groups, which had the same administered temperatures (Fig. 2B).
There is limited information on WSSV shedding in shrimp, but several previous studies have reported that WSSV can be detected in seawater, even when the number of viral copies in shrimp is approximately 102 to 103 copies per mg. These results suggest that the severity grade of G1 (threshold: 3.1 × 103 copies per mg) and G2 (threshold: 8.5 × 104 copies per mg) in shrimp sufficiently induce viral shedding and mortality, respectively. As viability of the virus could not be determined via real-time PCR, further studies are needed to clarify the correlation between histopathological analysis and molecular assays, including a combination of histopathological findings and viral viability determined using viability PCR assays.
Assessment of transmission risk in cooked, WSSV-infected shrimp
Viral shedding is essential for predicting virus loads in seawater and exposure levels for naïve individuals in contact with infected individuals (or exposed to contaminated water) and could be a useful tool for understanding the epidemiology of the disease. Results of other studies suggest that the viral shedding rate positively correlates with the severity and viability of the host. Therefore, the severity grades of shrimp and viral loads in seawater could be useful factors for determining disease severity and tracking disease outbreaks in epidemiological studies.
Similar to the results of injection challenges, the infection progressed most rapidly at 25 degrees-C. In the 20 and 30 degrees-C groups, mass mortality of the recipient groups occurred from 7 dpi, with greater than 105 WSSV genome copies per mg of viral copies in pleopods (abdominal legs). In the groups administered with 103 WSSV genome copies per shrimp, 100 percent cumulative mortality at 25 degrees-C was observed in both the donor and recipient groups. For the 20 degrees-C group, the infection of the recipient group was confirmed at 9 dpi, while the viral loads in seawater were exhibited as 102 to 103 WSSV genome copies per mL. Furthermore, the maximum viral copies of pleopods exhibited in the recipient groups coincided with the observation of mass mortality (30 and 25 degrees-C: 9 dpi; 20 degrees-C: 11 dpi; Fig. 3).
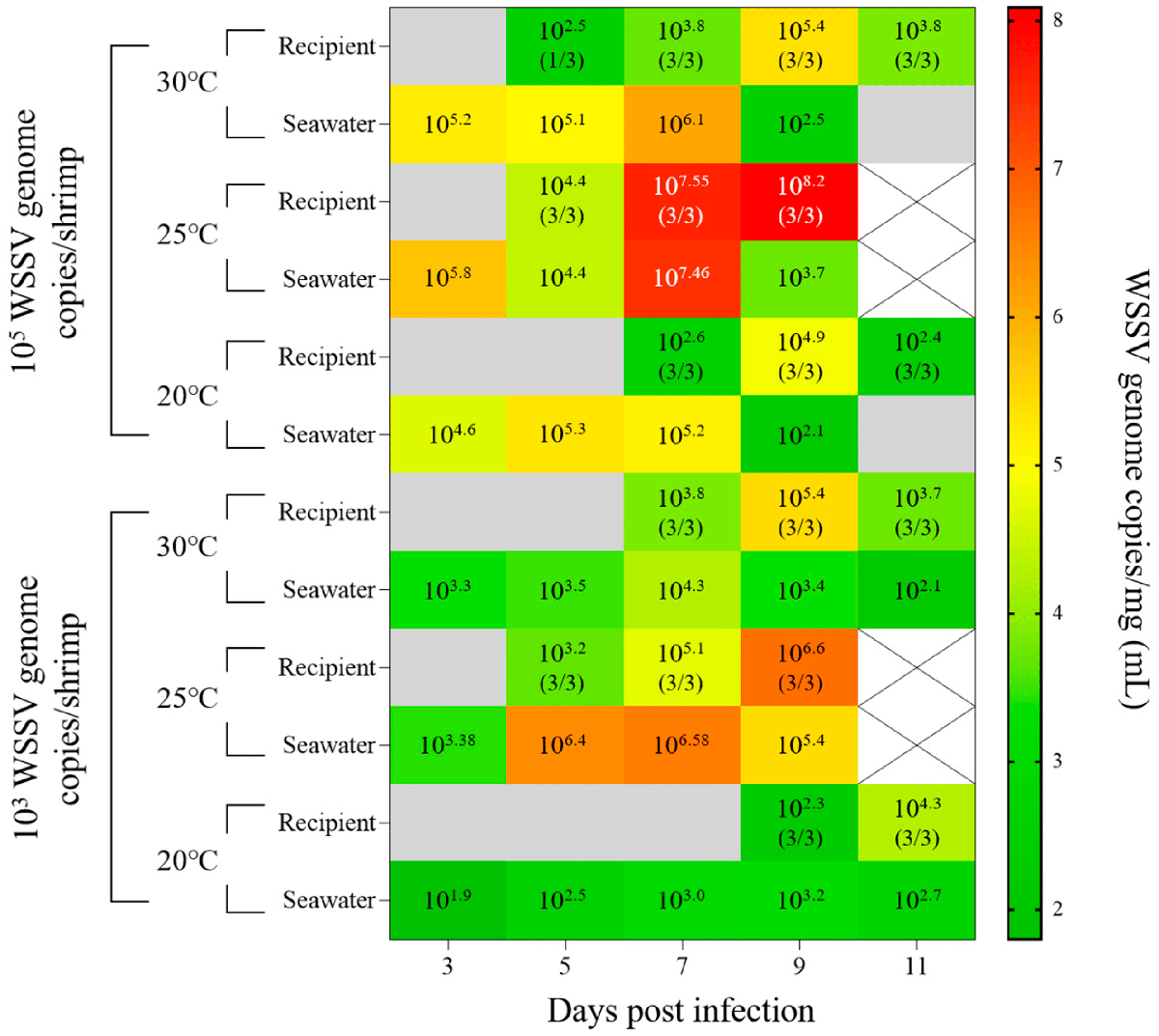
Based on these results, we found that despite the influence of water temperature on the waterborne transmission of WSSV, 105, 103, and 101 WSSV genome copies per mL in seawater could induce waterborne infections within approximately one, three, and seven days, respectively. These results suggest that waterborne transmission of WSSV is affected not only by the viral load in seawater, but also by the duration of exposure. Therefore, to better understand the waterborne transmission of WSSV, analyzing viral shedding is important for estimating its impact on naïve individuals.
In shrimp pond culture systems, the interaction between infected and naïve hosts could be crucial for sustaining the viability of WSSV. A previous study investigated the viability of WSSV in seawater and reported that WSSV could be preserved for up to 14 days in seawater under laboratory conditions. Based on this and other studies, viral loads in seawater could be maintained through interactions with hosts or vectors, and these interactions helped the WSSV remain viable for an extended period. To clarify the horizontal transmission mechanism of WSSV in pond culture systems, further studies are needed to investigate the more complex interactions, such as the influence of cannibalism and the dynamics of WSSV among host species, vectors and reservoirs.
Perspectives
We investigated the correlation between disease severity grade and clinical changes in the WSSV-infected shrimp. Our results revealed that a severity grade of G2 (cut-off value: 104.9 copies per mg) was sufficient to induce mortality in L. vannamei shrimp, and the infected shrimp with a severity grade of G1 (cut-off value: 103.5 copies per mg) was able to shed the virus.
Based on the correlation between disease severity grade and viral shedding rate, we determined a positive correlation between viral copies of the pleopods and viral shedding rate (y = 0.7076x + 1.414; p < 0.001). Furthermore, challenge tests mimicking natural conditions (immersion and cohabitation challenges) revealed that 105, 103, and 101 WSSV genome copies per mL of seawater could induce waterborne infection within 1, 3 and 7 days, respectively.
Our findings on the WSSV waterborne transmission model can aid in understanding the dynamics of WSSV in a pond culture system. Moreover, our results can be used to further elucidate the interactions between species during disease outbreaks and the spread of WSD in pond culture systems.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Kwang-Il Kim
Corresponding author
Department of Aquatic Life Medicine, Pukyong National University, Busan 48513, Republic of Korea[114,107,46,99,97,46,117,110,107,112,64,105,107,109,105,107]
Tagged With
Related Posts

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Australian shrimp growers demonstrate how to farm black tigers with WSSV knocking on their door
Successfully producing black tiger shrimp in the presence of White Spot Syndrome Virus shows the drive and toughness of shrimp producers Down Under.

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Health & Welfare
Improper use of PCR causes more harm than good
PCR testing is a valuable tool for detecting pathogens, when done correctly, but relying on it solely is not consistent with adequate biosecurity.



