Results show that nutrient-enriched, live lobster cockroaches (Nauphoeta cinerea) can enhance pearl arowana growth and pigmentation

Commercial scale production of insects such as mealworms and black soldier fly larvae has resulted in their growing use in aquafeed formulations. There is currently much interest in the use of cockroach-derived products in the food, feeds and pharmaceutical industry. Whether cockroaches can become the next aquafeed industry-relevant insect will greatly depend on the research conducted on its nutritive value.
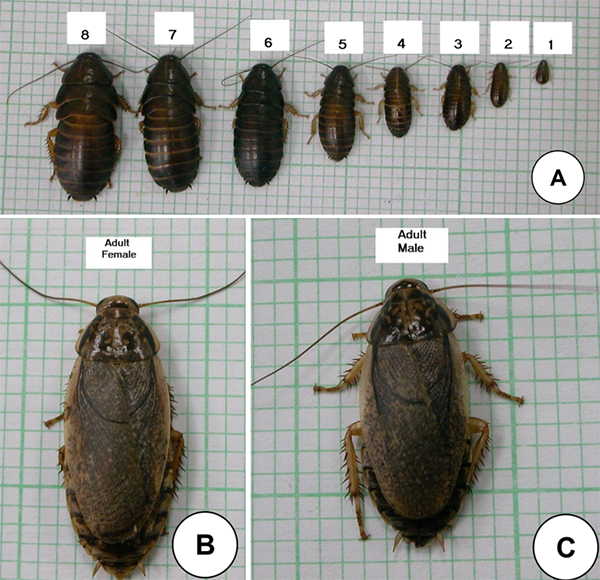
The lobster cockroach (Nauphoeta cinerea) is a tropical and subtropical species with a wide distribution. It is easy to maintain as it does not glide despite having wings in its adult stage. It belongs to the family Blaberidae and is the sole representative of its genus. Females are highly fecund and are ovoviviparous, incubating the ootheca (egg capsules) in the abdomen until the nymphs emerge. Nymphs undergo incomplete metamorphosis (eight instar stages) before emerging as adults, bypassing the pupal stage (Fig. 1). Nymphs have thin exoskeletons and are wingless.
In order to develop a sustainable mass production system, we conducted a comprehensive study to determine parameters such as fecundity, longevity and development periods of each life stage of the lobster cockroach. For the first time, the nutrient composition of the various life stages of the lobster cockroach was determined. Both the nymphal and adult stages, with different nutritional profiles, were then used in a feeding trial.
The production of nutritionally enhanced insects for use in aquafeeds is a research area of great interest and commercial potential. An attempt to produce “tailor-made” cockroaches in terms of protein and lipid content was investigated. Furthermore, lobster cockroaches enriched with carotenoids (astaxanthin) were also used to evaluate their impact on fish pigmentation.
Detailed information on the cockroach culture, development, reproduction, nutrient composition as well as other methodology used in the study can be obtained from the original publication by Ng et al.
The arowanas
The pearl arowana (Scleropages jardinii) is farmed alongside its more famous Asian species due to increased market demand. Unlike the Asian arowana, which are listed as threatened or endangered species, the pearl arowana is more accessible in the ornamental fish industry.
The term “arowanas” refers to two genera of fishes, including the genus Osteoglossum in South America and the genus Scleropages in Australasia. There are four species in the Scleropages genus, which includes two species from South Asia (S. inscriptus, S. formosus) and two species from the Australia/ New Guinea regions (S. leichardti, S. jardinii). The Asian arowana, S. formosus, is the most well-known and popular of the four species and is in high demand in the ornamental fish industry. Wild populations of the red, green and golden varieties of the Asian arowana are currently listed as “endangered” by the IUCN Red List of Threatened Species. Only farmed Asian arowanas are allowed to be traded, and their global trade is strictly regulated by the Convention on the International Trade in Endangered Species (CITES).
Arowanas have been called the “most expensive fish in the world” and are in high demand as a status symbol in many Asian communities. Arowana farming has been rapidly expanding in many countries to meet global demands from the aquarium fish trade. Unlike its Asian counterparts, the Australian or pearl arowana (S. jardinii) is not considered a threatened or endangered species. Very little information is known about the pearl arowana. Due to its accessibility, we decided to use the pearl arowana as the experimental fish model to represent the Scleropages genus.
Live food is the main nutrient input used in the captive farming of arowana. Using pelleted feeds has resulted in poor growth, low feed palatability, bone deformation and poor pigmentation in arowanas. Arowana farmers and hobbyists prefer to use live shrimp, fish, frogs, worms and insects. The high reproductive rate and low maintenance costs of insects make them a cost-effective live food in arowana aquaculture. In their natural habitats, arowanas have been reported to preferentially forage on insects. There is currently no comprehensive research nor published report on the use of live cockroaches in arowana feeding.
Thus, we investigated the effects of feeding live lobster cockroaches of different nutrient profiles and life stages on the growth performance, feed utilization efficiency, body indices and scale pigmentation in pearl arowana.
Evaluating live food and insect meal-based diets for larval rearing of sea trout
Arowana feeding trial
As arowanas are highly aggressive territorial fish, pearl arowana (mean weight = 32 g) were individually housed in aquaria (95 L) with its own water filtration and aeration systems. Each aquarium was tightly covered at the top with wire netting. A total of 24 fish were used in the study. During the one-week acclimation period, all fish were fed five live cockroaches (middle nymphal stage) daily from the stock cockroach population fed a commercial rodent feed.
The arowana feeding trial comprised three dietary treatments consisting of three different groups of live lobster cockroaches. The first dietary treatment used adult male cockroaches fed commercial rodent feed, resulting in body composition containing low lipid and high protein levels (3 and 30.1 percent, respectively; A-LLHP). The A-LLHP cockroaches were considered the control diet. Another group of cockroaches was fed our formulated cockroach feed (CF; 20.0 percent protein, 19.7 percent lipid) which allowed us to increase the lipid content of adult male cockroaches without affecting body protein content compared to the control group fed the rodent feed. This proprietary CF also contained 80 mg astaxanthin per kg feed as a carotenoid source (CAROPHYLL® Pink, DSM Nutritional Products Ltd., Thailand).
The second group of arowanas was fed adult male CF-fed cockroaches with mid-level lipid and high protein levels (6.1 and 29.7 percent, respectively; A-MLHP). The third group of fish was fed late nymphal cockroaches with high lipid and lower protein levels (10.3 and 22.9 percent, respectively; N-HLLP); these cockroaches were fed the rodent feed.
The protein and lipid content of all three groups of live cockroaches are shown in Table 1. Each group of cockroaches was fed to eight replicate fish once daily. Cockroaches were fed manually to each fish until apparent satiation and the number and weight of prey recorded. The feeding trial was conducted for 12 weeks.
Wing, lobster cockroaches, Table 1
| Composition (%) | Lobster cockroach1 | ||
|---|---|---|---|
| A-LLHP (control) | A-MLHP | N-HLLP | |
| 66.1 ± 0.4 | 65.0 ± 0.8 | 65.2 ± 0.0 | |
| Moisture | |||
| Dry matter | 33.9 ± 0.4 | 35.0 ± 0.8 | 34.8 ± 0.0 |
| Crude protein | 30.1 ± 0.5 | 29.7 ± 0.9 | 22.9 ± 0.2 |
| Crude lipid | 3.0 ± 0.1 | 6.1 ± 0.2 | 10.3 ± 0.3 |
1A-LLHP = adult male cockroaches fed the rodent feed which resulted in body composition containing low lipid and high protein levels.
A-MLHP = adult male cockroaches fed CF which resulted in body composition containing mid-level lipid and high protein levels. CF contains astaxanthin (80 mg/kg).
N-HLLP = late nymphal cockroaches fed the rodent feed which resulted in body composition containing high lipid and lower protein levels.
The source of live cockroaches had a significant effect on arowana growth performance (Table 2). The final body weight, percent weight gain and SGR were significantly higher (P < 0.05) in fish fed the N-HLLP cockroaches compared to the control A-LLHP group. Arowanas fed the A-MLHP cockroaches were not significantly different in growth performance compared to the A-LLHP or N-HLLP groups. Feed conversion ratio (FCR), food intake (number of cockroaches eaten per day) and protein efficiency ratio (PER) were not significantly different (P>0.05) among the different dietary groups. The best FCR and PER were observed in fish fed the N-HLLP cockroaches.
Wing, lobster cockroaches, Table 2
| Lobster cockroach2 | |||
|---|---|---|---|
| Parameter | A-LLHP | A-MLHP | N-HLLP |
| (control) | |||
| Average initial weight (g) | 31.52 ± 0.94 | 31.60 ± 0.91 | 33.74 ± 0.79 |
| Average final weight (g) | 57.47 ± 4.26a | 67.49 ± 8.69ab | 84.00 ± 8.32b |
| Weight gain (%) | 81.22 ± 8.57a | 109.44 ± 18.27ab | 151.85 ± 23.10b |
| Food weight (g/day) | 1.48 ± 0.10 | 1.51 ± 0.11 | 1.43 ± 0.20 |
| Cockroach (number/day) | 3.84 ± 0.10 | 4.56 ± 0.43 | 3.86 ± 0.23 |
| Specific growth rate (%/day) | 4.01 ± 0.07a | 4.14 ± 0.10ab | 4.38 ± 0.10b |
| Feed conversion ratio | 6.60 ± 0.91 | 5.94 ± 0.73 | 4.87 ± 0.76 |
| Protein efficiency ratio | 0.57 ± 0.05 | 0.67 ± 0.09 | 0.81 ± 0.09 |
| Survival (%) | 87.5 | 100 | 100 |
1Values are the mean ± S.E. of eight replicate fish. Mean values within same row with different superscripts are significantly different (P<0.05).
2 See Table 1 footnote for live cockroach description.
Scale chromatophore type and numbers

The location of the scale where the chromatophores (cells that produce color, with many types of pigment-containing cells found in many animals) were counted was the same for all fish. The number of xanthophores (yellow pigment cells) and melanophores (black pigment cells) were counted under a microscope. The melanophores were manually scored according to the degree of pigment dispersal based on the Melanophore Index (MI, developed by Hogben and Slome in 1931 and in use since the earliest studies of melanophore responses). Fully aggregated melanosomes were grouped into MI = 1 and fully dispersed melanosomes were grouped as MI = 5.
More melanophores in category MI=1 was observed in the scales of arowanas fed A-LLHP or A-MLHP whereas more melanophores in category MI=3 was observed in fish fed N-HLHP. However, these numerical differences were not significantly different. Scales of arowana fed the A-MLHP cockroaches had the highest count of melanophores. There was a significant difference among the treatments in terms of the total xanthophores counts. Only the scales of fish fed the adult cockroaches (A-LLHP or A-MLHP) exhibited these yellow pigment cells (Fig. 2). Scales of fish fed A-MLHP cockroaches showed the highest xanthophore count but were not significantly higher than the A-LLHP group. Xanthophores were not present in the scales of the N-HLLP group.
Conclusions and recommendations
In the present study, pearl arowana fed live lobster cockroaches at the late nymphal stages (N-HLLP) showed the best growth performance and feed utilization efficiency. Despite the lower protein content of the late nymphs (22.9 percent) compared to the adult male cockroaches (A-LLHP) with a protein content of 30.1 percent, pearl arowana showed higher growth, possibly due to the protein-sparing action of the high 10.3 percent lipid content in N-HLLP.
The importance of dietary lipids was further confirmed by the A-MLHP group, which showed better growth despite being fed adult cockroaches of similar protein content but double the lipid content compared to the A-LLHP group. Another potential reason for the better growth and FCR of arowana fed cockroach nymphs (N-HLLP) might be their higher nutrient digestibility compared to adult cockroaches. Based on the results of the present study, it is recommended that late nymphal stages of the lobster cockroach be used for better growth and feed utilization efficiency in arowanas and possibly for other fish species as well.
Color is one of the major determinants of the selling price of arowanas. In the present study, pearl arowana fed the A-MLHP astaxanthin-enriched cockroaches showed the highest melanophore and xanthophore cell counts on their scales. Interestingly, only the scales of arowana fed the adult cockroaches showed the presence of xanthophores, irrespective of whether the cockroaches were fed added astaxanthin or not in their diets.
For the first time, cockroaches were shown to be able to contribute to fish pigmentation by virtue of the natural pigments present in their cuticles. The thinner cuticle of the nymphal group (N-HLLP), presumedly with less pigments present, probably resulted in the absence of xanthophores in arowanas fed these nymphs. Nevertheless, melanophores were still observed in the scales of arowanas fed the cockroach nymphs. Therefore, it is recommended that adults and not the nymphal stages of lobster cockroaches be used as live feed for arowanas when the objective is to enhance fish coloration. Carotenoid-enriched adult cockroaches can be used to further enhance arowana pigmentation.
In this study, we attempted to produce “tailor-made” cockroaches by feeding them a formulated cockroach feed with higher lipid levels and added astaxanthin (A-MLHP, Table 1) which altered their nutrient profile. This resulted in arowanas showing higher growth performance and markedly more chromatophores on their scales. Fish were also observed to store more body fat which may be physiologically important during the breeding season. This study showed that live cockroaches can be nutrient-enriched to enhance arowana growth and pigmentation.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Wing-Keong Ng, PhD, FASc
Corresponding author
Retired Professor, Asian Aquafeeds Services, Penang, Malaysia[32,109,111,99,46,108,105,97,109,103,64,109,115,117,46,103,110,107,119]
-

Kean-Teik Koay, MSc
Director, Green2U group of companies, Malaysia
-

Chow-Yang Lee, PhD
Professor of Urban Entomology, University of California, Riverside, USA
Tagged With
Related Posts

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.

Aquafeeds
Is a ‘baby food’ bottleneck looming for aquaculture?
Global aquaculture, particularly farmed shrimp, depends on artemia for hatchery feeds. Supplies meet current needs, but growth will require alternatives.

Health & Welfare
A perspective on shrimp larviculture and liquid larval diets
A prominent shrimp farm and hatchery in Venezuela has tested microparticles, microencapsulated particles and liquid larval diets.

Health & Welfare
Colored LED lights can influence shrimp growth, water quality in biofloc culture
Pacific white shrimp reared with green and red lights grew much larger but showed little differences for survival, feed conversion or productivity.


![Ad for [Aquademia]](https://www.globalseafood.org/wp-content/uploads/2025/07/aquademia_web2025_1050x125.gif)