Professor Boyd: Examining the significance of an important production parameter

Electrical conductivity is frequently measured in aquaculture systems and taken as an indicator of the degree of mineralization (total ion concentration) of water. The principles and method of conductivity measurement were discussed in the previous article (part 1). The present discussion will be about the significance of the conductivity measurement in water.
Salinity, total dissolved solids
The salinity is a measure of the total ion concentration in water, and the majority of the total dissolved solids (TDS) concentration – usually at least 95 percent – is from dissolved ions. Therefore, conductivity is related to salinity and TDS concentration. This has resulted in the use of a modification of the conductivity meter to measure salinity and TDS concentration directly.
This is done by installing a factor into the circuitry of a conventional conductivity meter as a means of transposing conductivity readings to display salinity, total dissolved solids or both. Some meters have the capacity to display conductivity at one setting, and salinity, TDS concentration, or both at other settings.
Salinity and TDS concentration can be measured quite accurately by traditional methods. However, the meters for measuring these two variables – however convenient – have accuracy limitations. The conductivities of two waters of the same total ion concentration will rarely be identical. This results because different ions contribute differently to conductivity.
The factor for converting between conductivity and both salinity and TDS concentration is relatively constant (about 0.70) in ocean waters throughout the world. A good relationship also often exists between conductivity and TDS concentration (or salinity) in freshwater from a given physiographic region (Fig. 1). But, the factor for converting conductivity to TDS concentration or salinity varies from around 0.55 to 0.8 when considered across many different physiographic regions.
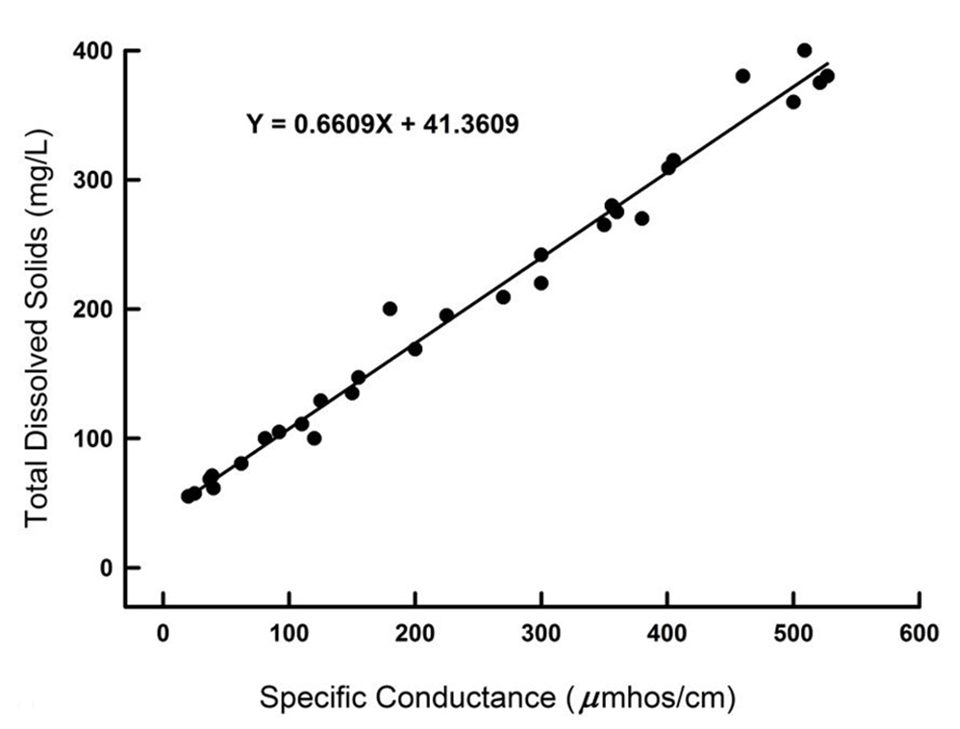
Moreover, the upper ends of estuaries are more similar to freshwaters than to ocean water in ionic makeup. No single factor could be expected to adequately convert the conductivity to salinity or to TDS relationship across all natural waters. Obviously, considerable error may result in the determination of either salinity or TDS concentration with modified conductivity meters.
The best approach is to use conductivity as an approximate indication of the degree of mineralization of water. In this way, no major mistakes in interpretation are likely, and inferences about a number of properties of water – especially the colligative properties – can be made. Colligative properties are changes resulting from dissolved substances that depend upon the total amount of all dissolved substances (solutes) and not upon the type of solutes in a water.
Osmotic pressure
A more important colligative property of water for aquaculture is the effect of greater conductivity on osmotic pressure. The osmotic pressure of a solution is the amount of pressure or force necessary to prevent the flow of water molecules across a semipermeable membrane (water molecules can pass the membrane, but solute molecules cannot) from a less concentrated solution to a more concentrated solution as illustrated in Fig. 2. This results because the pores in the membrane facing the concentrated side are more often blocked by ions than are those facing the less concentrated side – this result is a net movement of water to the concentrated side.
In applying the osmotic pressure concept (Fig. 2) to aquatic animals, body fluids of aquatic animals represent one solution, the surrounding water is the other solution, and the part of the animal that separates the two solutions can be thought of as the membrane. Freshwater animals have body fluids more concentrated in ions than the surrounding water; they are hypersaline or hypertonic to their environment. Saltwater species have body fluids more dilute in ions than the surrounding water; they are hyposaline or hypotonic to their environment.
The freshwater fish tends to accumulate water because it is hypertonic to the environment, so it must excrete water and retain ions to maintain its osmotic balance. Because the saltwater fish is hypotonic to the environment, it loses water to the environment. To replace this water, the fish takes in saltwater; but to prevent the accumulation of excess salt, it must excrete salt. Each species has an optimum salinity range. Outside of this range the animal must expend considerably more energy than normal for osmoregulation at the expense of other processes such as growth. Of course, if salinity deviates too much from optimum, the animal will die because it cannot maintain osmotic balance.
The effect of increasing salinity (and osmotic pressure) on common carp is shown in Table 1. The fish spend increasingly more energy for maintaining osmoregulatory balance as salinity increases. This results in less growth at higher salinity.
Boyd, conductivity, part 2, Table 1
| Salinity (ppt) | Food energy recovered as fish growth (%) |
|---|---|
| 0.5 | 33.4 |
| 2.5 | 31.8 |
| 4.5 | 22.2 |
| 6.5 | 20.1 |
| 8.5 | 10.4 |
| 10.5 | -1.0 |
Aquaculture relevance
Aquaculture species have a range of salinity (that can be estimated from conductivity) over which they grow best. In selecting a site at which to culture particular species, conductivity is an important consideration.
Conductivity also has an influence on corrosion of metals, and it can affect corrosion of aeration and other aquaculture equipment with metal parts. Higher electrical conductivity increases the potential of electron movement from anode areas to cathode areas on metal objects thereby increasing the rate of corrosion.
Electrofishing, a common practice in fisheries management for sampling fish populations, is also affected by the conductivity of the water. However, electrofishing is not often used in aquaculture. Those interested in the topic should read the nice explanation of the electrical power requirement of electrofishing. The URL is electrofishing.net/2016/03/04/power-transfer-theory-of-electrofishing-in-a-nutshell/.
The dissolved substances in water occupy space, and at the surface of natural waters some of the space in contact with the air is occupied by solute molecules. Vapor pressure of water is the pressure in the air resulting from water molecules when equilibrium is reached between water and water molecules in the air above it. Solutes reduce the vapor pressure of natural waters below that of pure water, because there are fewer water molecules in contact with the air than is the case with pure water.
The boiling point of water is increased, the freezing point of water is depressed, and evaporation rate is greater from freshwater than from ocean water as a result of the effects of solutes on vapor pressure.
Author
-

Claude E. Boyd, Ph.D.
School of Fisheries, Aquaculture and Aquatic Sciences
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Responsibility
Inorganic fertilization of production ponds
Nitrogen and phosphorus are the most important nutrients in fertilization of both freshwater and coastal ponds. Many factors that can affect the response of ponds to fertilizers are location-specific. Aquaculture pond managers will have to figure out the best procedure for a given location or even for an individual pond.

Responsibility
Reviewing the ‘near-magical’ properties of water
Understanding the hydrologic cycle, water’s unique physical properties and their behavior in aquaculture systems – phases, humidity, saturation, stratification, light reflection and refraction – is pertinent to properly manage water quality in production systems, according to world-renowned expert Dr. Claude Boyd.

Responsibility
What causes alkalinity changes in aquaculture waters?
Total alkalinity is an important variable in water for aquaculture systems, and its concentration frequently fluctuates over time in many culture systems.

Responsibility
Thoughts on improving responsible aquaculture intervention efforts
Certification programs presently are the best tool to assure that aquaculture products are responsibly produced. But these programs can be burdened by many superfluous requirements and their simplification by focusing primarily on the major issues would be a great improvement.

