Hatcheries in Japan, USA support research and commercial activities

Declining natural populations and increasing demand for flatfish in national and international markets have created a favorable economic environment for commercial culture of flatfish in the United States. Along the eastern seaboard of the U.S., two highvalue flatfish species of the genus Paralichthys – the summer flounder P. dentatus (Fig. 1) and the southern flounder P. lethostigma (Fig. 2) – are leading candidates for commercial grow-out in a variety of culture systems in inland as well as coastal areas.
Current status
In Japan, where commercial culture of the congeneric Japanese flounder (P. olivaeceus) is well established, commercial hatcheries rely on photothermal conditioning and natural spawning of broodstock to produce the large numbers of high-quality eggs needed by commercial grow-out operations and restocking programs. In the U.S, hormone-induced spawnings are used to produce summer flounder embryos for research and commercial applications.

Great Bay Aquafarms, New Hampshire, USA, the first U.S. commercial hatchery for summer flounder, was established in 1996. It currently can produce fingerlings to support grow-out operations. Broodstock management methods for southern flounder are less advanced, but significant numbers of high-quality eggs are now being produced for research through hormone-induced and natural spawnings.
Biology
Adults of both species emigrate from estuaries in the autumn and winter to spawn in offshore wintering grounds. Spawning migration disperses eggs over large areas of the continental shelf and avoids the extreme cold of shallow, near-shore waters.
Summer and southern flounder are serial spawners; they continuously mature and spawn egg batches over a protracted spawning season. During the spawning season, a mature adult female may possess oocytes of virtually all stages of development, from the early stages of yolk deposition (i.e., vitellogenic) to the fully mature, ovulated stages ready for expulsion.
While Japanese flounders produce up to 25 million eggs per female over a six-month period, summer and southern flounders release up to six million eggs per female during a spawning season. Relatively low fecundity and a continuous pattern of ovarian development pose significant challenges for broodstock managers with respect to availability and timing of seedstock production.

Controlled maturation
Researchers and commercial culturists rely largely on wild broodstock, and the potential for selective breeding remains untapped. Great Bay Aquafarms has recently initiated a selective breeding program for summer flounder and is producing progeny from F-1 hatchery-reared individuals.
Brooders are held year-round in small to mid-sized (2.5 to 10 cubic meters), controlled- environment tanks supplied with recirculating seawater (Fig. 3). These allow precise control of environmental conditions; efficient feeding; close observation of individual fish health, maturation, and behavior; and facilitate handling of individual brooders. Dark-colored tanks and low illumination levels (300 lux) are believed to reduce stress and promote voluntary spawning.
Varying stocking densities of 1.7 to 4.11 kilograms per cubic meter and sex ratios of 1 to 2 males: 1 female are used. Wild-caught brooders (especially southern flounders) are difficult to wean to pelleted feeds, so a variety of live (striped bass fingerlings, killifish, and shrimp) and frozen feeds (red hake, mullet, Atlantic silversides, lake smelt, mackerel, shrimp and krill) are fed, usually once daily to satiation.
Photothermal manipulation
In summer flounder, timing of gonadal growth can be precisely controlled by photothermal manipulation. When different groups of brooders were exposed to an accelerated natural or delayed artificial photothermal regime, the patterns of ovarian development (and of spermiation in males) under these regimes faithfully reflected the seasonal changes.
In all three groups, onset of vitellogenesis was associated with a declining photoperiod, even as temperature conditions ranged from 17 to 28 degrees-C. Therefore, declining photoperiod may trigger the deposition of yolk into the oocytes (i.e., vitellogenesis), while temperature may control the rate of yolk deposition and ovarian growth.
At Great Bay Aquafarms, photothermal conditioning is used to produce summer flounder seedstock yearround. For southern flounder, constant winter photothermal conditions are effective in extending the spawning period from January through April, at least two months beyond the natural spawning season, but additional studies are needed to demonstrate yearround seedstock production.
Hormone-induced spawning
The traditional method for hormone- induced spawning of flounder is by injection with carp pituitary homogenate (CPE; dose = 1 to 10 milligrams per kilogram), a crude extract containing gonadotropin as well as other hormones and water-soluble compounds. Developed in the mid-1970s, this method was used by researchers to produce small numbers of summer flounder embryos through the early 1990s.
Fish are checked for ovulation before being manually strip spawned, which can require up to two weeks. Timing of ovulation is estimated as when eggs first flow freely from the female upon slight pressure to the abdominal region in a “squeeze check.”
Ovulation is obtained within three to 14 days of the first injection, and multiple strip spawnings are obtained, although fertilization rates are variable and often low. Frequent handling can cause brooder mortalities.
Other hormones
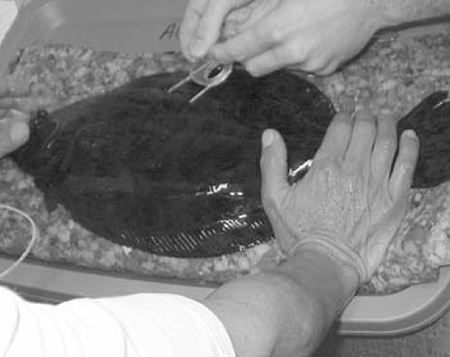
As interest in commercial culture of flounder increased, purified hormones were tested in an effort to develop more reliable, large-scale fingerling production methods. Human chorionic gonadotropin (250 to 1,000 IU per kilogram) injections were found only partially effective, producing small numbers of eggs with low fertility, or causing an “egg-bound” condition in which females hydrated, but could not be stripped of their eggs.
Intramuscular implantation of a cholesterol-cellulose pellet containing superactive analogues of mammalian gonadotropin releasing hormones (GnRHa, 50 to 100 milligrams per kilogram) has been most effective for inducing ovulation in summer and southern flounder to date (Fig. 4). In these serial spawners, the sustained release of GnRHa from the pellet stimulates the continuous release of gonadotropin from the fish’s own pituitary, causing the ovulation of successive clutches of eggs and repetitive spawnings.
Females with late vitellogenic stage oocytes (maximum diameters greater than 500 μ or mean diameters 258 to 456 μ) are responsive to pelleted GnRHa. These implants usually induce multiple spawnings, each approximately 24 hours apart, but fertilization rates are variable and generally decrease with successive spawns.
Natural spawning
Natural spawning of both the southern flounder and summer flounder without hormone induction has yielded small numbers of fertilized eggs. Recently, sustained natural spawning of southern flounder broodstock yielded commercially significant quantities of viable embryos. Wild-caught adults held in 4.7-cubic-meter tanks under a constant winter photoperiod of 10 L: 14 D from December through April produced a total of 49.4 million viable eggs.
Concurrent work on hormone-induced spawning revealed that natural spawnings yielded larger numbers of viable eggs per female, with higher fertilization and hatching success than hormone induced spawnings. Requiring little or no handling of broodstock, natural spawning was also much less labor intensive and time consuming than hormone-induced spawning. Minimal trauma during capture, varied diet, high water quality in brood tanks, tank size and black color, and low illumination levels were factors believed to contribute to successful natural spawning.
Natural Vs. induced spawning
While natural spawning minimizes time and labor requirements and improves egg quality, reliability and control are of great concern to the broodstock manager. Hormone-induced spawning can be timed to suit the needs of the culturist, who may opt to compensate for lower egg quality with concurrent hormone-induced spawning of multiple females.
As natural spawnings become more prevalent through longer-term acclimation and domestication, a combination of natural and GnRHa-induced spawnings of photothermally conditioned fish may help supply the large numbers of eggs necessary to support commercial hatcheries.
Constraints

Whether hormone-induced or natural spawning approaches are used, a fundamental problem in the controlled reproduction of both the summer and southern flounder is the variability in number of eggs, fertility and hatching rates, and egg quality among females. Higher and more predictable rates of ovulation, as well as fertilization and hatching, are needed to lower costs of seed production.
During strip-spawning (Fig. 5), variable fertilization rates can be attributed, in large part, to improper timing. Stripping can disturb the ovulation process when performed prematurely, or cause a rapid loss of viability inside the ovary after ovulation if stripping is delayed (over-ripening).
Timing of ovulation
Commercial aquaculturists at Great Bay Aquafarms have developed an elegant alternative to the “squeeze check” to judge the timing of ovulation. This method, which takes advantage of the flounders’ flattened body shape, is being used as a research tool with southern flounder.
Flounders are placed on a transparent “light table” illuminated from below. As light is transmitted through the fish, the size and location of the gonads can be seen through the body wall (Fig. 6). In females, stages of ovarian development are based on the size, shape, and degree of translucency of the ovaries, which increase as oocyte hydration and ovulation progress.
Limited spermiation

Limited availability of spermiating males and their low milt volumes has also been problematic during hormoneinduced spawning of summer and southern flounder. Furthermore, the same hormone treatments used on females have been ineffective for inducing spermiation in males. Research is needed to clarify the endocrinological basis for this observation and to find alternative hormonal therapies for inducing spermiation.
Compressed vitellogenesis
In these serial spawners, the period of vitellogenesis is compressed as compared to species that release a single clutch of eggs. It is likely that inappropriate nutritional and temperature regimes during vitellogenesis disrupt normal egg development. This leads to inadequate transfer of nutrients into the eggs, affecting their biochemical composition and quality. Methods to improve biochemical egg quality – through optimizing nutrition, environmental conditioning regimes, and hormone dose and dose rates – are needed.
Conclusion
Increasing market demand for flatfish favors its commercial culture. Summer and southern flounder are two high-value flatfish species with much potential to meet this demand through commercial grow-out in a variety of culture systems. Much progress has been achieved in the controlled maturation and spawning of these species, but some constraints remain. The list of publications used in this review is available from the senior author.
(Editor’s Note: This article was originally published in the October 2000 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Wade O. Watanabe, Ph.D.
University of North Carolina at Wilmington
Center for Marine Science
7205 Wrightsville Ave.
Wilmington, NC 28403 USA[117,100,101,46,108,105,119,99,110,117,64,119,101,98,97,110,97,116,97,119]
-
George C. Nardi, M.S.
Great Bay Aquafarms
153 Gosling Rd.
Portsmouth, NH 03801 USA
Tagged With
Related Posts

Responsibility
Southern flounder culture at Texas Parks and Wildlife Department
The Texas Parks and Wildlife Department’s southern flounder stock enhancement has stocked more than 400,000 fingerlings into Texas bays and estuaries.

Health & Welfare
Cobia culture in recirculating systems
A unique pilot project supported by northern Chile’s biggest power-generating company and the Undersecretary of Fisheries and Aquaculture is raising cobia in a recirculating aquaculture system in the desert.

Intelligence
Marine fish culture in Brazil
Marine fish farming in Brazil appears promising. A new cobia research network will support industry growth through standardization, development of technology and training.

Intelligence
Southern Brazilian flounder
Work on the reproduction and larval rearing of southern Brazilian flounder supports the feasibility of flounder culture. Further research will improve weaning and grow-out techniques for the species.



