Clearing up several misconceptions about the role of vibrio bacteria in shrimp diseases

This is an update of a brief article I wrote a few years ago summarizing the challenges that shrimp farmers face when it comes to dealing with vibrio infections. Bacterial infections of farmed shrimp are quite common and likely the major cause of mortality in farmed shrimp. Vibrios are certainly a major actor, but they are not the only cause by a long shot. Far too often the failure to take proactive measures results in crashes by reactively dealing with the diseases once they occur.
A partial list of species of vibrio that have been associated with disease outbreaks in shrimp at various points in the production cycle is presented in Table 1. The reader should bear in mind that, within a given species, there may be many strains that cannot cause disease, and in fact, virulence is rare. It is also important to appreciate that most vibrio outbreaks are due to opportunistic bacteria. Animals are weakened by stressors which can include viral pathogens, and this is what makes them susceptible. The industry should be focusing on minimizing the presence of stressors rather than trying to selectively control vibrio loads. Doing one without the other is very often counterproductive.
Newman, Vibriosis, Table 1
| Species | TCBS reaction (Green-G or Yellow-Y) | Disease/Comments |
|---|---|---|
| V. alginolyticus | Y | Zoea syndrome, Septic Hepatopancreatic Necrosis (hatchery and grow out), shell disease |
| V. anguillarum | Y | Shell disease (juvenile and adults) |
| V. harveyi | Y | Luminescent vibriosis (eggs and larvae), Early Mortality Syndrome(EMS) or Acute Hepatopancreatic Necrosis (AHPND) (larvae, juvenile and grow-out) |
| V. parahaemolyticus | BG, G (there are Y strains-rare)* | Early Mortality Syndrome (Acute Hepatopancreatic Necrosis) (larvae, juvenile and growout), Zoea syndrome, Septic necrosis (hatchery and growout), shell disease |
| V. vulnificus | G (there are Y strains) | Septic necrosis (hatchery and grow-out), shell disease |
| V. splendidus | Y/G | Shell disease, luminescent vibriosis (eggs and larvae) |
| V. fluvialis | Y | Shell disease |
| V. campbellii | Y | Zoea syndrome, Septic necrosis (hatchery and grow-out), Early Mortality Syndrome (Acute Hepatopancreatic Necrosis) (larvae, juvenile and grow-out) |
| V. mimicus | G | Zoea syndrome, Septic necrosis (hatchery and grow-out), shell disease. Very similar to V. cholerae. |
| V. owensii | G | Early Mortality Syndrome (Acute Hepatopancreatic Necrosis) (larvae, juvenile and grow-out) |
| V. orientalis | Y | Luminescent vibriosis (eggs and larvae), also known as V. bivalvicida |
| V. mediterranei | Y | Luminescent vibriosis (eggs and larvae), |
| V. logei | Y | Luminescent vibriosis (eggs and larvae), |
| V. penaeicida | * | Summer syndrome in grow-out |
| V. nigripulchritudo | * | Summer syndrome in grow-out |
As Table 1 shows, a wide range of specific strains of selected species of vibrios can cause similar disease problems. This does not mean that every member of the species is of concern or that those that are of concern are best dealt with by attempts to exclude every member of the genus from production systems.
(Editor’s note: This article is adapted from the original source.)
Vibrios
Vibrios are gram-negative bacteria [that do not hold a specific, distinguishing stain], curved-shaped rods, and most can grow in the absence of oxygen (termed facultative anaerobes). This is not the preferred method of growth, but it allows them to thrive in environments that are not optimal. They prefer temperatures that are at or above 15 degrees-C (59 degrees-F).
Vibrios are a component of most aquatic ecosystems, and the majority are found in seawater and brackish water, although V. cholerae is also found in fresh water. These bacteria are highly evolved, having two chromosomes that allow them to be genetically quite flexible. The exact number of species is a moving target, as more are being identified regularly. Estimates are that there are more 150 species, with likely thousands of strains.
Most are benign and cannot cause disease unless present at densities that can only be achieved by growing them in the lab. They are ubiquitous in water and colonize many aquatic animals including fish, shrimp and crabs, among others, as well as algae, planktonic forms of a variety of organisms and suspended organic matter. They readily form complex assemblages, known as biofilms, which allow them to produce disease and ensure environmental persistence. They are instrumental in the biodegradation of chitin. Table 1 above lists the majority of those that have been implicated in shrimp disease. Most, if not all, of these species have multiple strains, many of which are not pathogenic. There are two broad categories that pathogens fall into:
- Obligate pathogens cause disease when present. A single cell can be enough to initiate a disease process. A very small number of bacteria are enough to set off a pathological process that kills the host. Typically, the animal has no ability to defend itself against infection and they succumb quickly. These are relatively rare.
- Opportunistic pathogens cause disease when other factors weaken the host. Most bacteria that kill shrimp fall into this category. In the absence of stressors, these can be benign. They can be present at very high levels and yet not cause any problems. Most bacterial disease in farmed shrimp is due to opportunistic pathogens.
While there is no doubt that vibrios are the major cause of bacterial disease outbreaks in farmed shrimp, the role of stressors cannot be ignored.
Misconceptions about the role of vibrios in shrimp disease
Misconception #1: Vibrios are all bad bacteria, and no other bacterial genus is.
Many other species of bacteria have been implicated in disease outbreaks in farmed shrimp. Most are probably opportunistic, as much as most of the vibrios are. Some of the genera that have been implicated are Aeromonas, Pseudomonas, Streptococcus, Bacillus, Photobacterium, Pasteurella and Shewenella, among others. Furthermore, the fact that most bacteria cannot be cultured on agar media leads to the inference that there may be many other bacterial pathogens that have as of yet not been identified. Most bacteria that kill shrimp are acting opportunistically.
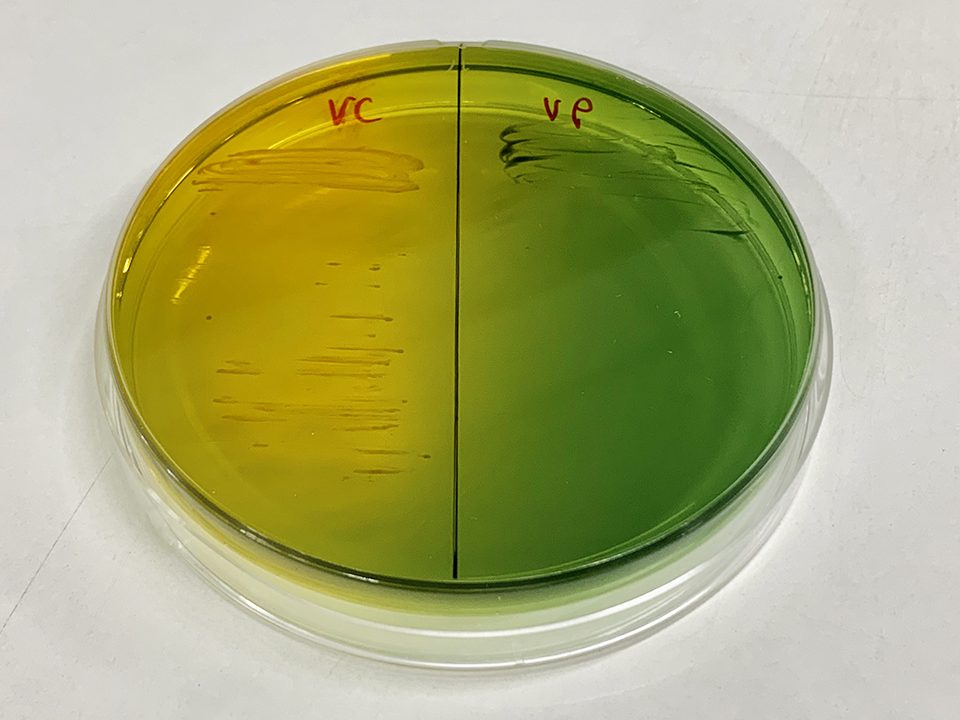
Misconception #2: The bad vibrios are green on Thiosulfate-citrate-bile salts-sucrose agar (TCBS) and the good ones are yellow.
Thiosulfate-citrate-bile salts-sucrose agar (TCBS) is a selective media that was developed some years ago for the isolation and selection of vibrios. Not all vibrios grow on it, and the commonly used distinction of colony color on the agar – which is misrepresented as being related to virulence – reflects the ability to use the sugar sucrose. There is no correlation between this and the presence of toxins or the ability to produce disease, yet this misconception persists. Many assert that if you can keep the TCBS green colonies (cannot digest sucrose) out of the hatchery and the farm, you do not need to be concerned with the impact of any TCBS yellow colonies. They state that these are benign. But the most virulent vibrio I have ever seen was a strain of Vibrio alginolyticus that was TCBS yellow and caused an outbreak in Belize: sucrose fermenting vibrios (yellow) on TCBS can be highly virulent.
Misconception #3: Responsible biosecurity requires efforts to moderate vibrio loads.
Vibrios serve a very important role in the degradation of chitin. Chitin is the second-most abundant biomolecule in nature after cellulose and it makes up the cell walls of crustaceans, fungi and insects. Where there is chitin in aquatic ecosystems there will be vibrios. Since chitin is a major structural component of all crustaceans, the vibrios are naturally associated with its presence. Getting rid of vibrios opens niches for other potential pathogens, and there is no guarantee that these potential pathogens will not be worse than any vibrio you may have eliminated. Efforts to mitigate the impact of vibrio loads should be general in nature and not geared towards reducing loads to the point where gaps in niches allow entry by other bacteria, just as capable of causing disease, to dominate.
Misconception #4: Farmed animals will be healthier and stronger if there are low levels of vibrios in a production system.
Disease is the result of an interaction between a host animal, the environment, and the potential pathogen. Animals that are produced in a manner that minimizes the stress that they are under have the best chance of thriving. Strong, healthy animals are much more likely to realize their genetic potential than stressed animals are. Unless the vibrios present are obligate pathogens and are present at threshold levels (levels needed to ensure disease in healthy animals), efforts to control them in an absolute manner will not protect animals from disease. Other non-vibrio pathogens will cause disease.
Misconception #5: Stress is not cumulative.
Stress comes in many different forms. When shrimp and other cultured aquatic species suffer from anoxia [low oxygen supply] or other stressors and recover, one cannot assume that they will recover to the point they were at before the event. Animals respond in many ways to stress, and how depends on what the stressors are and how long they are present. The same reaction occurs when animals have been exposed to toxic materials. They may appear to be just fine, but this exposure can have long-lasting impacts on the animal’s steady internal conditions or homeostasis. Many believe that it is acceptable to allow the animals to be exposed to sublethal levels of toxins: no animals are dying, so they do not see a problem. But weakened animals are more susceptible to opportunistic pathogens and lower threshold levels of obligate pathogens.
Responsible farming methods must take this into account. The goal should be to produce crops with little to no stress, not to see how much stress one can get away with.
Misconception #6: Polymerase chain reaction (PCR) screening provides an absolute assurance that animals are free of the pathogens that they are being tested for.
PCR is a powerful tool that was never intended to be used in the way that the shrimp farming industry uses it. Standard PCR testing is not quantitative, it is “yes” or “no.” The presence of a presumptive pathogen does not mean that there is an active disease process occurring or that it will occur. And the absence does not mean that it is not present. It only means that the sample was negative.
PCR can also be quantitative, and this is known as real-time PCR. It can be used to follow the growth of a pathogen (obligate and opportunistic) in a susceptible population. If the levels increase with time and this is occurring concomitantly with a degradation of animal performance, then it is safe to assume that these are potentially related. Although PCR results can be quite useful, they have one serious shortcoming: when one screens animals based on statistics (i.e., taking a subsample of a small percentage of a population and testing these animals as a window into what is transpiring in the population as a whole) there is always a chance that the pathogen is present and that the screening missed it (false negatives).
Only by following animal performance in the field can one be sure that the PCR results for a population are consistently valid. Furthermore, if one does not ensure that the way the animals are being tested is consistent with the known behavior of the potential pathogen of interest, false negatives will occur. Perhaps the best example is for the virus that causes white spot disease [White Spot Syndrome Virus, WSSV]. This virus does not grow well at warmer water temperatures, and it thrives at cooler temperatures. If you don’t test animals that are held at cooler temperatures, you will always get false negatives.
Another example would be that of strains of V. parahaemolyticus that carry the PIRa and PIRb toxins [that cause Acute Hepatopancreatic Necrosis, AHPND] and may not be detectable by standard PCR without enrichment or amplification. The toxin might be present as evidenced by damage to susceptible tissues, but PCR testing comes up negative. Samples of suspect origins of the bacteria must be cultured in broth for 12 to 24 hours before PCR testing is conducted. In many instances, samples, which were found to be negative by PCR testing initially, can eventually be positive.
Conclusions
It is challenging enough to be consistently successful in farming shrimp, but it is even harder when there is a huge amount of misinformation being widely circulated as factual. The key to successful and sustainable production is to see these for what they are, and to not allow them to interfere with the reality.
While there is no doubt that vibrios are the major cause of bacterial disease outbreaks in farmed shrimp, the role of stressors cannot be ignored. Farmers spend a great deal of money and time trying to control vibrios when they routinely ignore stressors. Some stress is always inherent in any farming paradigm, and genetic selection can be quite useful in generating lines of animals that are more tolerant than the wild type. Indeed, this is the basis of domestication.
But until farmers accept the reality that preventable stress is what is allowing opportunistic bacteria to impact their farmed shrimp, these bacteria will continue to exact a huge toll on the global farming industry. Trying to eliminate them in an all-or-none manner is more than likely just going to lead to other challenges.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Stephen G. Newman, Ph.D.
President and CEO
Aquaintech Inc.
Lynnwood WA, USA
www.probioticsaquaculture.com[109,111,99,46,104,99,101,116,45,110,105,45,97,117,113,97,64,109,119,101,110,103,115]
Tagged With
Related Posts

Health & Welfare
Bacterial pathogens in biofilms pose health risks in recirculating systems
Recirculating aquaculture presents an increased potential for pathogenic bacteria to become established in the system through the formation of biofilms.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.

Health & Welfare
Aquaculture disease diagnosis is detective work
Those charged with determining the underlying cause of a disease outbreak have a wide variety of detective tools at their disposal.

Health & Welfare
Biosecurity: Not just a word
Most aquaculture farms rely on general strategies for biosecurity that implement protocols learned from others. Establishing effective biosecurity, however, can be complicated due to multiple interacting variables.



