Results show that immunization of tilapia broodstock with TiLV vaccines could be a potential strategy for the prevention of this important disease

Tilapias are one of the most important freshwater fish species farmed globally and are now cultured in more than 140 countries. The Tilapia Lake Virus (TiLV), also known as Tilapia tilapinevirus, is one of the most significant infectious agents causing relatively high mortality and economic losses for tilapia farmers. The mortality rate in natural TiLV outbreaks ranges from 20 to 90 percent, while cumulative mortalities from experimental infection range from 66 to 100 percent. The virus can infect fertilized eggs, yolk-sac fish, fry, fingerlings and adult fish. Recent studies have reported that TiLV can also be transmitted vertically from infected broodstock to their offspring.
Vaccination is an effective strategy to prevent infectious diseases in aquaculture, and several vaccines have been described for the control of TiLV in tilapia. Most recently, water-based inactivated vaccines prepared with heat-killed or formalin-killed TiLV were shown to provide good levels of protection in juvenile tilapia. It has also been reported that TiLV vaccines can induce both humoral [through antibodies] immunity and cell-mediated [without antibodies] immunity.
This article – adapted and summarized from the original publication [Mai, T.T. et al. 2022. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines 2022, 10(2), 167] – reports on a study to investigate levels of TiLV-specific antibodies in Nile tilapia broodstock after immunization with either heat-killed vaccine (HKV) or formalin-killed vaccine (FKV), and the role of these antibodies in protecting juvenile tilapia against TiLV through passive immunization. We also assessed the transfer of maternal antibodies from vaccinated broodstock to their fertilized eggs and larvae.
Study setup
We hypothesized that Nile tilapia (Oreochromis niloticus) broodstock immunized with a TiLV inactivated vaccine can mount a protective antibody response and passively transfer maternal antibodies to their fertilized eggs and larvae. To test this hypothesis, three groups of tilapia broodstock, each containing four males and eight females, were immunized with either a heat-killed TiLV vaccine (HKV), a formalin-killed TiLV vaccine (FKV).
Booster vaccination with the same vaccines was given three weeks later, and mating took place one week thereafter. Broodstock blood sera, fertilized eggs and larvae were collected from six to 14 weeks post-primary vaccination for measurement of TiLV-specific antibody (anti-TiLV IgM) levels. In parallel, passive immunization using sera from the immunized female broodstock was administered to naïve [not previously exposed to the virus] tilapia juveniles to assess if antibodies induced in immunized broodstock were protective.
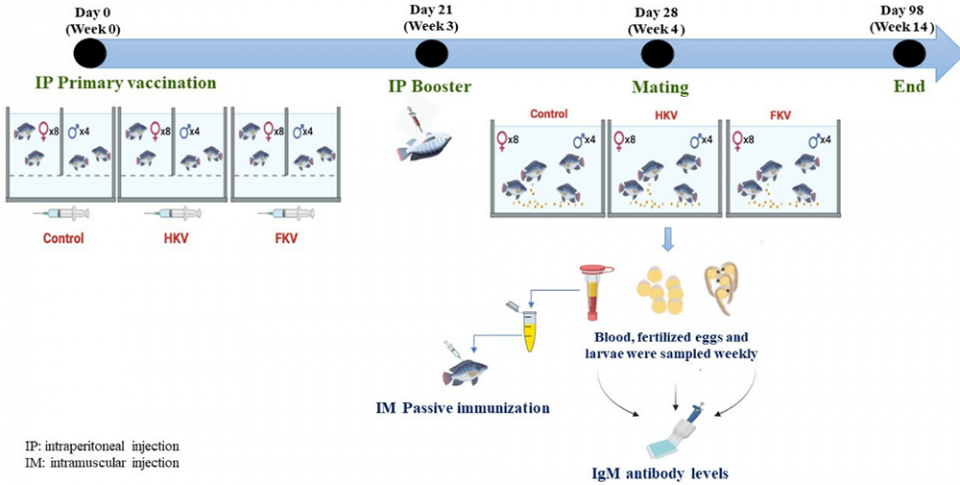
For detailed information on the experimental design and fish; animal husbandry; vaccine preparation and dosages; immunization, breeding and sampling, and other topics in this study, refer to the original publication.
Results and discussion
In a previous and related study, vaccination of tilapia juveniles with HKV and FKV resulted in a significant increase in systemic TiLV-specific IgM and a high level of protection against TiLV challenge (relative percent survival, RPS = 71.3 to 79.6 percent). However, the persistence of a specific antibody was not evaluated.
In our current study, we used the same vaccination protocol for the tilapia broodstock, using double doses of antigen per fish as in our previous study, for both primary immunization and the booster vaccination. Relatively high levels of TiLV-IgM were detected from six to 14 weeks post-primary vaccination, suggesting that both HKV and FKV elicited relatively long persistence (98 days) of TiLV-IgM in vaccinated broodstock. This finding is consistent with a previous observation in tilapia juveniles challenged with TiLV, where a specific antibody response was maintained for six to 16 weeks post-infection.
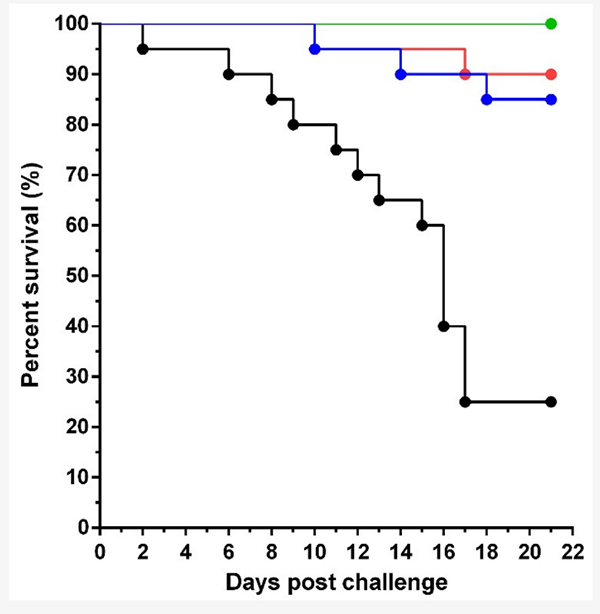
The reduction in TiLV load during the course of infection, which decreased to undetectable levels in surviving fish by the end of the experiment, reinforces the presumed role of protective antibodies in virus clearance. Theoretically, these antibodies could be capable of removing TiLV from the body of the fish by various mechanisms, and several studies have shown that passive immunization can protect fish from viral infection. Because tilapia broodstock are usually kept in the hatchery for three to five years, vaccination would be an effective strategy to prevent TiLV infection in the broodstock, minimizing economic loss and maintaining good health of the broodstock during the breeding period.
Our results provide evidence that maternal antibodies from TiLV-vaccinated tilapia broodstock are transferred to their offspring. Interestingly, these antibodies were found to be protective during passive immunization in tilapia juveniles challenged with the virus. This suggests that anti-TiLV antibodies may not only help to reduce the risk of infection in broodstock but may also reduce the risk of vertical TiLV transmission. Several studies have reported that vaccination of broodstock is an effective strategy to enhance the maternal transfer of immunity from mother to offspring and reduce the risk of vertical transmission of the pathogen.
Higher levels of TiLV-IgM were found in the fertilized eggs of the group vaccinated with HKV than in those of the fish vaccinated with FKV, suggesting that HKV is more promising for successful maternal vaccination. However, TiLV-IgM transfer only persisted for one to three days post-hatch and was undetectable by seven and 14 days post-hatch. Because the TiLV challenge was unsuccessful at the larval stage of tilapia, we did not evaluate passive antibody protection in the offspring. However, these findings suggest that maternal antibody transfer in larvae does not last long and may be insufficient to protect offspring after one to three days post-hatch.
Sizing up TiLV and its potential impact on tilapia production
This result agrees with the results observed in other fish species like grouper vaccinated against Nervous Necrosis Virus, NNV, where specific antibodies were found to gradually decrease within 48 hours after hatching. Such short persistence can be explained by the gradual decline in IgM during yolk-sac absorption observed in tilapia, and other fish such as European sea bass (Dicentrarchus labrax) and Atlantic salmon (Salmo salar). Therefore, in addition to vaccination, biosecurity measures remain essential to prevent the introduction of pathogens into tilapia hatcheries, especially during seed production.
Perspectives
Results of our study have shown that vaccination of tilapia broodstock with HKV and FKV elicits a protective antibody response against TiLV, and that these antibodies can be transferred to the fertilized eggs and larvae to induce maternal immunity. HKV appears to have greater potential than FKV for maternal transmission of antibodies. However, protective antibodies had a short persistence in the larvae leaving a gap between maternal immunity and immunocompetence. Further vaccination is therefore likely to be needed to protect fish from TiLV infection during this gap, as well as for later stages of development.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Ha Thanh Dong, Ph.D.
Corresponding author
Aquaculture and Aquatic Resources Program, Department of Food, Agriculture and Bioresources, School of Environment, Resources and Development, Asian Institute of Technology, Khlong Nueng 12120, Thailand[104,116,46,99,97,46,116,105,97,64,103,110,111,100,116,104]
Related Posts

Health & Welfare
Evaluating genetic parameters for resistance to Tilapia Lake Virus in Nile tilapia
Study estimates genetic parameters for host resistance to Tilapia Lake Virus in a Nile tilapia and finds TiLV resistance is highly heritable.

Health & Welfare
Experimental transmission of Tilapia Lake Virus in broodstock
Study evaluates the experimental transmission of Tilapia Lake Virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs.

Health & Welfare
Hyperthermia can boost innate immune system in juvenile fish
Hyperthermia, or heat shock treatment, is an unconventional approach to pathogen control in fish aquaculture that appears to have potential to improve disease resistance in fish like tilapia and barramundi.

Responsibility
Giving a lift to tilapia farmers in Myanmar
The U.S. Soybean Export Council’s farm demonstrations, farmer education efforts and formulated feeds are boosting tilapia production in rural areas of Myanmar, where indigenous carp are more commonly raised.



