Regular additions necessary to maintain algal blooms

Phosphorus is a key nutrient that regulates the growth of phytoplankton in aquaculture ponds. One reason is that phosphorus is naturally in short supply relative to its requirement by phytoplankton. Freshwater phytoplankton usually must concentrate phosphorus more than any other essential nutrient, and marine phytoplankton normally concentrate only nitrogen more than phosphorus. In addition, when phosphorus is applied to ponds, it tends to precipitate directly from the water or be quickly adsorbed by bottom soil.
Phosphate fertilizers
Phosphate fertilizers applied to ponds stimulate phytoplankton growth and increase the base of the food web. However, much greater aquaculture production can be achieved through the use of feed to supplement natural productivity. Phosphorus from uneaten feed and the feces of culture animals can cause excessive phytoplankton growth and associated degradation of water quality.
The most common phosphate fertilizer used in aquaculture is triple superphosphate. It is made by treating rock phosphate, an ore obtained from deposits of marine origin, with phosphoric acid. Triple superphosphate typically contains 20.1 percent phosphorus, primarily as water-soluble monocalcium phosphate. When applied to pond water, granular fertilizer dissolves into calcium and phosphate ions.
Phosphorus in ponds
Aquafeeds usually contain 1.0 to 1.5 percent phosphorus. Most phosphorus in aquafeed is in organic combination, but some feeds are supplemented with calcium phosphate. Roughly 25 percent of the phosphorus in aquafeeds is harvested in aquaculture biomass. The remainder is in uneaten feed and feces that microbially decompose and release phosphate, or metabolic excretions of culture animals.
Phosphate ions can be absorbed rapidly by phytoplankton. Following pond fertilization, a large portion of the applied phosphorus can be taken up by phytoplankton cells within a few hours. However, phytoplankton have life spans of just one or two weeks, and when they die, the phosphorus in their cells is quickly mineralized to soluble phosphate.
Phosphate ions not absorbed by phytoplankton can be absorbed rapidly by bottom soil. The combined effect of phytoplankton uptake and bottom soil adsorption is a rapid disappearance of phosphorus from pond water following the addition of phosphate fertilizer (Fig. 1). Where bottom soils are acidic, phosphorus is sequestered as aluminum and iron phosphates. In ponds with more alkaline soils, phosphorus is fixed as calcium phosphate.
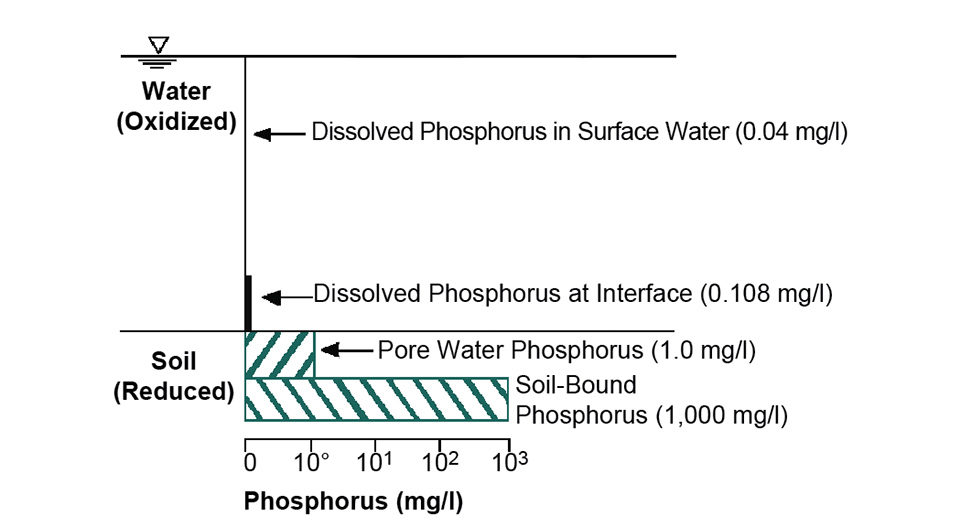
Studies at Auburn University revealed that about two-thirds of the phosphorus applied to research ponds in fertilizers and feeds over a period of 23 years accumulated in the soil. Nevertheless, the soil in these ponds still had a great capacity to adsorb phosphorus. Typical concentrations of phosphorus in the water and bottom soil of ponds are illustrated in Fig. 2.
In pond water containing high concentrations of calcium and a moderate to high pH, calcium phosphate can precipitate directly without the direct involvement of bottom soil. Direct precipitation is a common phenomenon in the water of ponds in arid regions and those filled with brackish water or seawater.
The phosphorus in bottom soils is not highly soluble, and the equilibrium concentration between phosphorus in soil and phosphorus in water is low – often only a few micrograms per liter. Soil phosphorus availability is greatest at a pH of about 6.5 to 7.0. In this pH range, concentrations of iron, aluminum, and calcium – the substances that react with phosphate to form highly insoluble compounds – are low. Thus, fertilized ponds with acidic bottom soil should be treated with agricultural limestone to raise pH and increase the availability of phosphorus applied in fertilizers.
Liquid fertilizers
Liquid fertilizers are more effective than triple superphosphate and other granular fertilizers in pond fertilization. Granules of phosphate fertilizer can settle to the bottom before completely dissolving. Although the granules continue to dissolve, their close contact with soil particles favors the rapid adsorption of phosphorus by the soil.
Liquid fertilizers can be completely dissolved in water to assure greater uptake by phytoplankton. Although they must be applied at two- to four-week intervals to maintain phytoplankton blooms, phosphorus application rates can be reduced to 25 to 50 percent of those necessary with granular fertilizers.
Liquid fertilizers are considerably more expensive than granular ones. A satisfactory alternative to liquid fertilizers is to place granular fertilizer in a container of pond water at a 1:20 ratio, let the mixture stand for 30 minutes, and then stir it vigorously before splashing it over the pond surface.
Phytoplankton management
Regular additions of phosphorus every two to four weeks are necessary to maintain phytoplankton blooms. Daily feeding can also provide a continuous input of phosphorus that supports the blooms. At high feeding rates, however, blooms can become excessively dense and lead to low nighttime dissolved oxygen levels.
High phosphorus inputs also favor the dominance of phytoplankton by blue-green algae. These organisms often form undesirable surface scums and are subject to massive die-offs. The decay of dead algal cells can cause dissolved oxygen depletion and massive fish kills. Blue-green algae also produce compounds that, when absorbed by culture species, cause off flavor in edible tissue. Some blue-green algae even produce compounds toxic to fish and shrimp.
In ponds where culture animals are offered feed, feeding rates may reach a point where low nighttime dissolved oxygen depletion becomes problematic. In channel catfish culture, this occurs at daily feeding rates of 30 to 40 kg per ha. Mechanical aeration can lower the risk of dissolved oxygen depletion and allow higher feeding rates and more fish production. Additional relief from overabundant phytoplankton has been achieved by introducing water into ponds to flush out phosphorus, other nutrients, and phytoplankton. However, this practice is discouraged because it increases the potential for pond aquaculture to pollute natural waters.
Applications of 25 to 50 kg per ha of burnt or hydrated lime, or greater applications of agricultural limestone to ponds at intervals of a few days have been promoted as a means of removing phosphorus from water and reducing phytoplankton abundance. Experiments at Auburn University have revealed possible benefits of lime treatment for phytoplankton control.
However, the application of soluble aluminum and iron compounds appears to have greater potential for removing phosphorus and controlling phytoplankton growth. Recent studies found that applications of ferric chloride or ferrous sulfate at concentrations as low as 6 mg per liter could reduce soluble reactive phosphorus concentrations by more than 80 percent. Slightly higher concentrations of aluminum sulfate also were effective.
Additional research and farm trials on the chemical removal of phosphorus are needed. However, care must be exercised in treating water with these substances, for excessive amounts can cause low pH and potentially toxic concentrations of iron or aluminum.
Pond liners
Aquaculture ponds in sandy areas sometimes are fitted with plastic liners to lessen seepage. In lined ponds without soil, phosphorus concentrations become very high. A dense layer of dead phytoplankton often accumulates on pond bottoms and causes an anaerobic zone. Phytoplankton communities also tend to be unstable, periodically blooming to great density and then crashing.
A 5- to 10-cm-deep layer of soil containing at least 10 to 15 percent clay over all or part of the liner usually will stabilize water quality. Of course, if lined ponds are operated as heterotrophic systems rather than green water systems, high phosphorus concentrations are not troublesome.
(Editor’s Note: This article was originally published in the July/August 2007 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA[117,100,101,46,110,114,117,98,117,97,64,49,101,99,100,121,111,98]
Tagged With
Related Posts

Aquafeeds
A look at India’s fish feed industry
India's fish-farming industry makes limited use of modern feeds, providing potential for the feed sector to grow. Commercial feeds are predominantly used for pangasius farming, followed by a rising popularity in carp culture.

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Aquafeeds
Animal byproduct concentrates useful tools in formulation
With the market volatility of fishmeal, as well as rising sustainability concerns, the aquaculture industry is seeking sources of protein, such as animal byproduct concentrates, to substitute for fishmeal.

