Scott Nichols concludes his series about GM foods with a deeper dive into transgenic animals
Editor’s note: This is part 4 of a four-part series on genetically modified (GM) foods. Previous articles by the author covered GM labeling; a comparison of conventionally vs. genetically engineered changes in agriculture; and regulatory and civil society views of agricultural change.
The state of genetically engineered farm animals is not as advanced as it is for crop plants. In the United States, there are eight genetically engineered plants in commercial production but no transgenic animals. The most notable advances in transgenic animals lie outside agriculture.
Most transgenic animals are for scientific research and medicine
The first transgenic animal was a mouse developed in 1974, though it wasn’t until 1981 that three research groups produced fertile transgenic mice. Beyond being mere laboratory curiosities, rodent species have been generated that are model systems of human diseases. Amongst many diseases studied this way are cancers, Parkinson’s disease, obesity, heart disease, diabetes, arthritis and substance abuse. These engineered mice have proven useful both for gaining fundamental understanding of diseases as well as for testing experimental treatments.
In biological research on fish, transgenic medaka (Japanese rice fish) and zebrafish are used to study developmental processes and the responses of fish to environmental contaminants.
Though outside the realm of food production, farm animals such as goats and rabbits are used for drug production. Mammals make the proteins used as pharmaceuticals in proper active forms. Microbes can also make pharmaceutical proteins but, often, those proteins are made in inactive forms.
Genetically modified animals in brief
There a pipeline of genetically engineered animals in various stages of development but only one has regulatory approval.
The single approved product is a modified Atlantic salmon produced by AquaBounty Technologies. Its AquAdvantage salmon contain an introduced growth hormone gene that is active throughout the whole year. Because the native salmon growth hormone gene’s activity diminishes during winter months, AquaBounty salmon grow to market weight in markedly less time than non-engineered salmon. AquaBounty salmon has received regulatory approval in the United States and Canada but, as yet, it isn’t available for sale in either country.
Other examples of transgenic animals in development are:
- Channel catfish, tilapia and carp with engineered growth hormone genes.
- Channel catfish genetically engineered to resist bacteria.
- Pigs with an introduced phytase Phytase helps pigs utilize phosphorus from their diets more efficiently with twin benefits of reducing phosphorus waste from farms and reducing feed costs for farmers.
- Pigs and sheep with an introduced gene that enhances omega 3 production. Animals with this gene produce about four times as much of the essential omega 3 fatty acid EPA. Moreover, they contain about a third less of the inflammation-inducing omega-6 fatty acids.
- Cows with an introduced lysostaphin gene. Lysostaphin is a protein that kills Staphylococcus aureus, which is a costly and endemic disease in dairy cattle.
Balancing the need for an increased food supply and protecting the environment
In late 2015, back-and-forth discussions about genetically engineered salmon were much like earlier (and still ongoing) discussions about genetically engineered crop plants. Opinions were rife. For those not favorably disposed to genetic engineering, the U.S. Food and Drug Administration (FDA) was lax to a fault both by having inadequate criteria for oversight and poor implementation of the regulations that do exist. If you were favorably disposed, the FDA’s ruling followed the most thorough analysis of a food ever undertaken.
In civil society, the discussion about genetically engineered crops is fraught because, figuratively, we’ve put the cart before the horse. We have innumerable answers. Unfortunately, they are not in response to carefully articulated questions about how we meet the demands on our agriculture/aquaculture system and the ethical imperatives we employ to assess advances.
So, just what is it we want?
As we approach mid-century we certainly need more food. Furthermore, our need goes beyond total calories; the nutritional content of our food should improve as well. Both desires should be achieved without increasing agriculture’s environmental impacts and, indeed, it would be advantageous if new traits and practices were improvements over those we have now.
With the ask of our aquaculture and agriculture systems to produce more and more nutritious food in ways that do not degrade the planet’s ability to continue to provide food, we need ways to assess new agricultural traits and varieties irrespective of how they are produced ― via genetic engineering or conventional breeding.
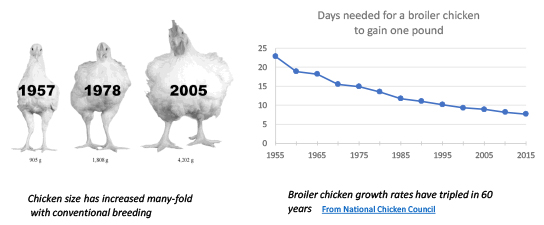
This last point is important. The figures show growth rates are being changed for our agricultural animals with both conventional breeding techniques and genetic engineering. Our attention should be on both.
In an earlier article I suggested five criteria we might use to judge the desirability of newly developed crop plants. In the best of all worlds they would:
- Increase food and nutrition security
- Provide improvements over current practices
- Provide solutions to environmental problems
- Engender biodiversity retention
These are good as far as they go but animal welfare needs to be considered. Animal welfare is an area that has, for very good reasons, attracted tremendous attention and any attempt to abstract that to a line or two is likely to be lacking. Nonetheless, here are two points I would add to the four above.
- We should not accept new traits that diminish quality of life for farm animals.
- We should strongly favor those that don’t.
Lastly, there are major differences between farming animals on the land versus the ocean. Fish that escape farms are difficult to find and recapture. If, however, a cow wanders off your farm, finding it is rather straightforward. (Indeed, even as a non-farmer, it was quite easy for me to spot my neighbor’s cows ― five of them actually ― that visited my yard one morning.)
Furthermore, it is more likely that escaped fish would breed with wild fish than would be the case with escaped terrestrial farm animals and nearby wild animals.
Simply put, the potential environmental concerns posed by modified fish are greater than those posed by modified terrestrial farm animals.
Weighing the many considerations is difficult. A compelling win in one of them would be good. So far, for fish, I see no compelling win.
Genetically engineered animals ― marketplace perspective
The first traits engineered into crop plants improved on-farm performance by reducing inputs of either labor or capital. They were successful and provided meaningful benefits to farmers.
There were two basic reactions to the new foods. One was indifference, a shrug and a resounding “meh.” The other was a more negative reaction that concluded changes were not demonstrably better but actually worse.
In important aspects, those first product introductions were inauspicious. Consumers saw no tangible improvement in their food. Perhaps the new crops slowed the rate of increases in food prices but that’s not the sort of thing that makes your heart race with anticipation as you walk into the grocery store. In the end, those involved in producing the seeds, crops and finished products didn’t come remotely close to making a what’s-in-it-for-me? case to consumers.
With the first genetically engineered animal approved for agricultural production, history is repeating itself. Just as with crops having modified input traits, the benefits of raising the AquAdvantage salmon would accrue to farmers. Because the quicker growing AquAdvantage salmon would be grown only in on-land tanks, farmers would produce more fish with the capital invested in their farms.
When all is said and done, though, it’s hard to see how farmers’ capital asset utilization will mean much to consumers.
There are, however, traits that could be environmentally attractive. Some that come to mind are: Early Mortality Syndrome (EMS)-resistant shrimp, sea lice-resistant salmon and bivalves that detoxify domoic acid. Each of these would represent important improvements in aquaculture. They would make seafood safer and reduce its environmental footprint.
No matter how good environmental advances, achieved through whatever means, the industry needs to show they meet consumer needs. Addressing the what’s-in-it-for-me? question is not pandering to unseemly self-indulgence. In many ways, it is the seminal question. The criteria I summarized above certainly do matter and we must show how they matter to consumers. Seafood providers need to make the case for a new 4-Ps of seafood marketing: Profit through Palate, Planet and People.
Author’s note: I have previously stated thoughts on AquaBounty salmon and have written about or been quoted on those thoughts elsewhere. You can read a few examples here, here or here. For a very good article that offers thoughts that differ from my writing, please see here.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Scott Nichols
Scott Nichols is the founder of Food’s Future, LLC a consultancy that helps clients build new ventures that accelerate the expansion of sustainable aquaculture. He is a regular speaker and writer on the role of aquaculture in our future food supply and is a member of the board of directors at the Aquaculture Stewardship Council.
Tagged With
Related Posts

Innovation & Investment
Aquaculture Exchange: Scott Nichols, Food’s Future
Scott Nichols speaks to the Advocate about the launch of his own consultancy, Food’s Future, about groundbreaking innovations at Verlasso and about the role of aquaculture in a rapidly changing world — one in need of collaboration and new ideas.

Responsibility
On GM foods, part 1: Let’s move this unproductive conversation forward
In the first part of a series on genetically modified foods, independent advisor Scott Nichols discusses the simplistic decision behind product labels and the more complex question of what could and should be the outcomes of its use.
Responsibility
On GM foods, part 2: Let’s talk about what truly matters
With entrenched positions on GM foods, it is difficult to change the conversation, but change it we must. A perpetually contentious climate doesn’t serve us well and we must abandon the current paradigm to define a new framework that facilitates more useful discussions.

Innovation & Investment
On GM foods, part 3: Let’s decide where we’re going
To assess the role of genetic engineering in agriculture, we should first state what we want from our agricultural systems. Agriculture, and by extension the future of aquaculture, lacks a succinct direction.