Complete elimination of fishmeal and fish oil is possible without affecting growth, health or consumer acceptance

According to the Food and Agriculture Organization of the United Nations, there was a more than 2,000-fold increase in global production of largemouth bass (Micropterus salmoides) from 1999 to 2018. The most recent data suggest that around 435,000 metric tons of largemouth bass were farmed in 2018, with a value over (U.S.) $1.24 billion. Almost all (99.5 percent) farm-raised largemouth bass originates from China, with the majority being reared in ponds. Critical to future growth and sustainability of this aquaculture, however, will be the development of diets that are less dependent on fishmeal and other marine resources.
Despite this, and as with other carnivorous species, there has been a reticence to move away from fishmeal-based feeds due to reports suggesting that its replacement depresses feed intake and growth, and has detrimental effects on animal health and welfare (DOI: 10.1016/j.oneear.2019.10.018). Another facet that works against replacing marine ingredients in aquafeeds is the suspicion that alternative ingredients may adversely affect product quality. This includes issues such as fillet yields; body and flesh pigmentation; processability (e.g., smoking, freezing, shelf-life); and most important, consumer acceptance (safety, traceability, texture, flavor).
As part of the F3 Future of Fish Feed initiative – which has the goal of eliminating marine ingredients from aquafeeds – we designed and carried out studies to examine the possibility of removing marine ingredients from largemouth bass diets, and results were published in the peer-reviewed International Journal of Fisheries and Aquatic Studies. The alternative proteins used in the present studies included a poultry by-product meal (PBM), hydrolyzed soybean meal (SBM) and a SB protein concentrate. Furthermore, the trials explored the possibility of replacing fish oil using an omega-3 rich algal product (AlgalPrimeTM), thereby achieving the F3 goal of eliminating fishmeal and fish oil from the diet.
Experience with other, essentially carnivorous fish (DOI: 10.1016/j.aquaculture.2009.11.003; 10.1371/journal.pone.0186705) teaches that this challenge can, with due care, be conquered. Furthermore, previous studies with largemouth bass have demonstrated that substantial proportions of fishmeal can be substituted without undue effects on growth, feed conversion, protein digestibility, or body composition (DOI: 10.111/jwas.12415; 10.111/j.1365-2095.2007.00533.x).
https://www.aquaculturealliance.org/advocate/testing-diets-without-fishmeal-and-fish-oil-for-kampachi/
We also evaluated three other concerns in these trials. First, the health of experimental animals was assessed with reference to structural changes to the intestine and liver, while the spleen was appraised for the staining intensity of melanomacrophage centers, MMC [microscopic structures possibly involved in activation of adaptive immunity].
Second, customer endorsement of the final product was examined through a user-based taste trial; and third, substantiation of the fact that animals were reared using marine resource-free feeds was assessed using stable isotope ratio mass spectrometry. The former issue has obvious implications whereas the latter two have become of increasing importance to consumers more attuned to social concerns surrounding sustainability. These same individuals are prepared to pay more for organic and environmentally sound products (DOI: 10.3390/su11061577; 10.1016/j.marpol.2020.104176).
Setup of studies
Two trials were undertaken, the objective of both being not only to evaluate fishmeal- and fish oil-free feeds, but also to reduce cost of the feed to make new formulas more acceptable to producers. The studies were completed using recirculating aquaculture systems at the Aquatic Research Laboratory, Prairie Aquatech (Brookings, South Dakota USA; Fig. 1). Water quality (dissolved oxygen, DO2: ~8 mg/L; temperature, 28-30 degrees-C; salinity, ~3.5 mg/L; pH ~8.4; total dissolved solids ~4 g/L; NH3, 0.33 mg/L; NO2, ~0.5 mg/L; NO3, ~25 mg/L) values throughout the studies was suitable for largemouth bass cultivation (Tidwell. J., Coyle, S., Bright, L.A. (Editors). Largemouth bass aquaculture. Wellcome Trust, London, U.K., 2018; 272 pp).

First study
The first study assessed the response of fish – growth and health – to the different formulations (Table 1), while the second study examined fish taste and traceability. Diets included a commercially available feed (COM) presently used by LMB producers (Classic Bass®, extruded, floating; protein/fat: 48/18; Skretting Tooele, Utah, USA) and three experimental feeds, varying in protein and lipid levels.
McLean, largemouth bass diets, Table 1a
| Ingredients | FMC | FMF | FFF |
|---|---|---|---|
| Algae meal-1 | – | – | 0.06 |
| Hydrolyzed soy meal-2 | – | 0.15 | 0.15 |
| Corn gluten meal | 0.0816 | 0.0816 | 0.0816 |
| Whole cleaned wheat | 0.2219 | 0.254 | 0.227 |
| Poultry meal-3 | 0.2082 | 0.2562 | 0.2562 |
| Fishmeal-4 | 0.263 | 0 | 0 |
| Vitamin premix | 0.005 | 0.005 | 0.005 |
| Lysine | 0.0135 | 0.0197 | 0.0197 |
| Methionine | 0.0034 | 0.0064 | 0.0064 |
| Choline chloride | 0.006 | 0.006 | 0.006 |
| Mineral premix | 0.0025 | 0.0025 | 0.0025 |
| Stay C (L-Ascorbat-2-Mono) | 0.002 | 0.002 | 0.002 |
| Soy oil (non-GMO) | 0.031 | 0.03 | 0.027 |
| Fish oil, menhaden-5 | 0.03 | 0.03 | 0 |
| Monocal phosphate, 21% | 0 | 0.0135 | 0.0135 |
| Taurine | 0 | 0.01 | 0.01 |
| Threonine | 0.0019 | 0.0031 | 0.0031 |
| Soybean meal (non-GMO)-6 | 0.11 | 0.11 | 0.11 |
| Lecithin | 0.02 | 0.02 | 0.02 |
| TOTAL | 1 | 1 | 1 |
1-Profine VF®, Dupont Nutrition and Biosciences, 2-AlgaPrimeTM, Corbion Inc., San Francisco, CA., 3-MrFeed Pro50 S®, Menon Renewable Products Inc., Escondido, CA., 4-Tyson River Valley Animal Foods, Texarkana, AR., 5,6-Daybrook Fisheries, New Orleans, LA., 7-South Dakota Soy Processors, Volga, SD.
McLean, largemouth bass diets, Table 1b
| Proximate composition | FMC | FMF | FFF | COM-7 |
|---|---|---|---|---|
| Dry matter | 92.27 | 92.1 | 90.28 | 92.86 |
| Crude protein | 46.8 | 42 | 41.5 | 50.8 |
| Fat (acid hydrolysis) | 13.7 | 14.5 | 15 | 17.2 |
| Ash | 9.25 | 7.2 | 7.39 | 7.37 |
| Fiber (crude) | 1.03 | 1.53 | 1.17 | <0.20 |
| Phosphorus (total) | 1.54 | 1.26 | 1.26 | 1.18 |
1-Profine VF®, Dupont Nutrition and Biosciences, 2-AlgaPrimeTM, Corbion Inc., San Francisco, CA., 3-MrFeed Pro50 S®, Menon Renewable Products Inc., Escondido, CA., 4-Tyson River Valley Animal Foods, Texarkana, AR., 5,6-Daybrook Fisheries, New Orleans, LA., 7-South Dakota Soy Processors, Volga, SD.
All experimental fish were fed to apparent satiation three-times daily and group weighed at three-week intervals, with feeding rates following commercial feed tables. Feed consumption was monitored for determination of feed conversion ratio (FCR = grams fed/grams gained) and mortalities recorded daily. At the end of the first trial fish were weighed and measured individually and a sub-sample from each treatment (n=3 fish/tank, 12 per treatment) euthanized (MS-222; Tricaine S, Western Chemical Inc., Ferndale, Wash., USA) and bled via caudal venipuncture for hematocrit determination (Fisher Scientific, Pittsburgh, Pa., USA).
These fish were also used for tissue analyses, including collection of liver, intestine, and spleen for histological evaluations and determination of somatic (= weight of tissue (g)/weight of fish (g) x 100) and visceral fat (VFI = weight of fatty tissue (g)/weight of viscera (g) x 100) indices. Other performance indicators included:
- Biomass gain, Fulton’s condition factor (k = wt/L3 x 100000)
Relative growth rate = (wt – wi)/100 , wi * where wt was final weight and wi initial weight; and
- Specific growth rate = ((ln(final weight/length) – ln(initial weight/length))/number of days
Tissue samples from liver, spleen, head kidney, proximal and distal intestine were prepared for histological analyses using standard methods. Analyses of these samples were used to determine impacts, if any, of the experimental diets on targeted organs. Liver lipid, glycogen and glucose were quantified using standard methods.
Second study
For the second trial, 60 to 64 fish from each treatment group were randomly distributed into eight larger tanks (densities of 3.11 ± 0.29 kg per square meter; biomass 2970.3 ± 275.7 grams; Fig. 1). Fish were weighed as a group every three weeks for a further 18 weeks, and their performance monitored in terms of survival and FCR.
A preliminary taste trial was undertaken using fish derived from the commercial and F3 dietary groups. Twenty-five active consumers of LMB were sent color-coded samples and asked to prepare their fish using plain methods. Each was then asked to establish whether there were differences in taste, texture, or aroma between the samples.
Finally, the muscle (taken dorsal to the midline between the second dorsal and caudal fins) of three fish per treatment were also sampled for carbon (δ13C) and nitrogen (δ15N) isotope ratios, as well as strontium analysis. Samples were collected and sent to the Marine Biological Laboratory, Woods Hole, Mass., USA, Stable Isotope Laboratory, where they were processed and analyzed.
All statistical analyses were performed using JASP software (JASP Team, 2019, Version 0.11.1) at the α=0.05 level of significance. Differences between treatment means were examined by one-way ANOVA and significant differences isolated using Tukey’s studentized range (honestly significant difference) test. Any potential tank effect or associated handling/treatment stress was assumed to be identical for each dietary group.

Results
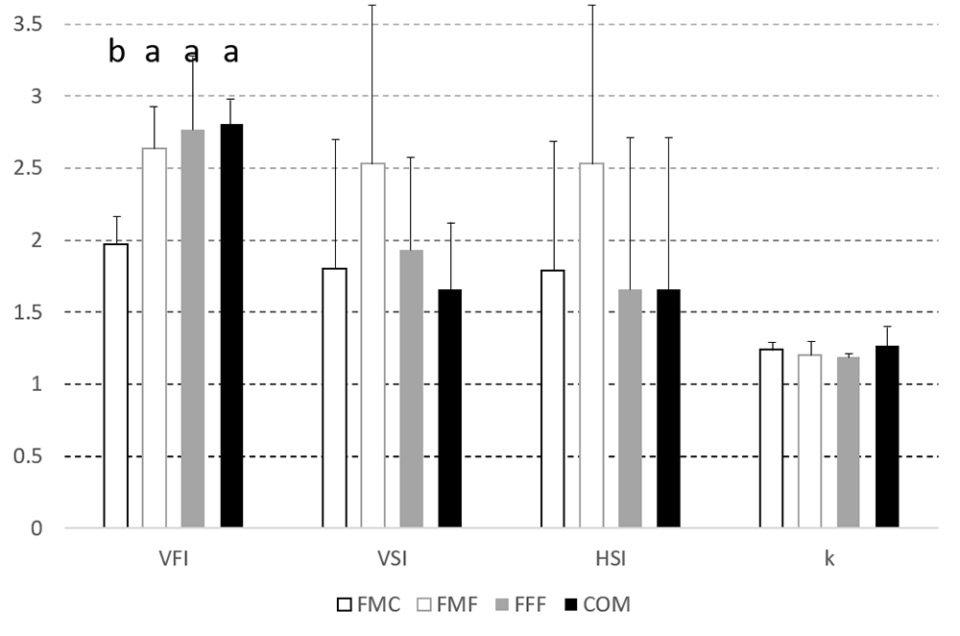
During the first trial, which evaluated the growth response of largemouth bass to the experimental feeds, fish weight increased between 200 and 230 percent, while length increased between 119 and 130 percent. There were no differences in weight, weight specific growth rate, length, length specific growth rate or Fulton condition factor, k [which relates fish length and weight, and is considered a health index], although the FCR for the COM diet was lower (P < 0.05) than the FFF diet.
Likewise, hematocrit [proportion of red blood cells by volume in the blood], visceral somatic index, VSI [weight of the viscera as a percentage of total body weight] and splenosomatic index, SSI [weight of the spleen expressed as a percentage of total body weight] did not differ between treatment groups but visceral [body fat in abdomen that cushions organs] fat index, VFI, was lower in the FFF diet group when compared to fish fed on the FMF feed. Hepatosomatic index, HSI [ratio of liver weight to total body weight; used as a measure of the energy reserves of an animal, especially in fish] was highest in the FMC group, with livers being larger (P < 0.05) than those observed in the COM fed fish. Liver vacuolization, glucose, lipid, and glycogen levels and (non)-MMC hemosiderin [an iron storage complex within cells] were equivalent across all groups.
In the second trial, there were no differences between dietary groups for feed consumption, but by trial end, the largemouth bass fed the COM diet were heavier (P < 0.05) with percent increase in biomass shown in Fig. 2. The FCR values also differed with the order FFF 1.95±0.09c > FMF 1.95±0.13b,c > FMC 1.78±0.06b > COM 1.67±0.02a. VSI, SSI, HSI and k did not differ between groups at trial end (Fig. 3). However, visceral fat index was lower in fish fed the FMC diet when compared to all other feeds (P < 0.05).
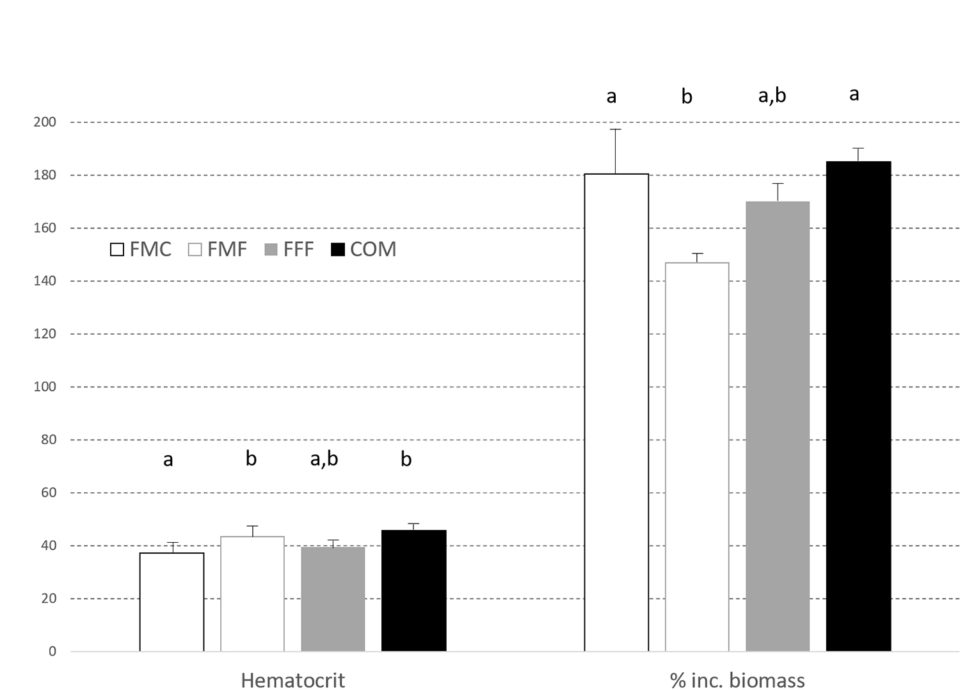
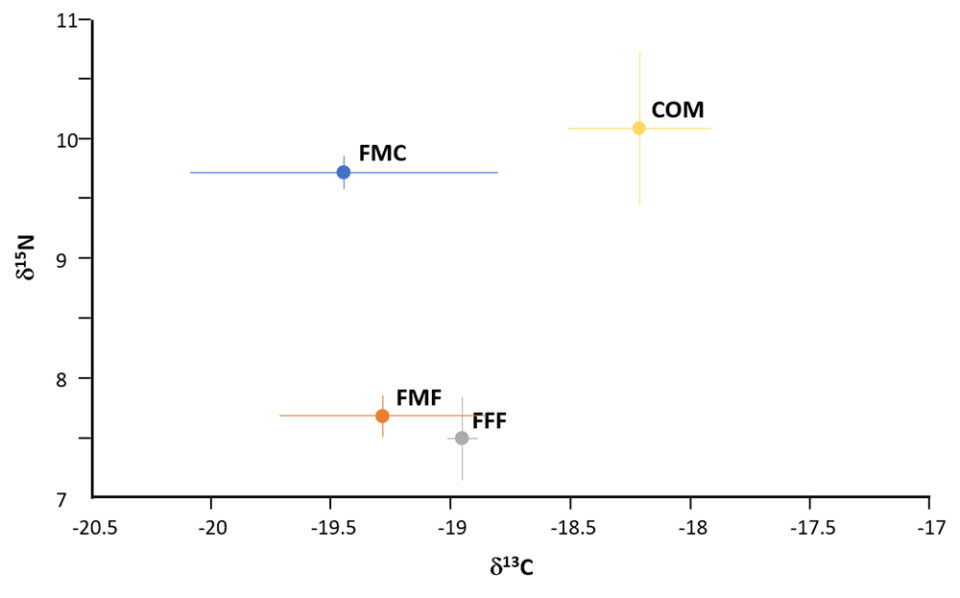
Hematocrit was similarly impacted by diet as depicted in Fig. 2. Stable isotope ratio mass spectrometry [a technique to measure the relative abundance of isotopes (atoms of the same element that have different numbers of neutrons but the same number of protons and electrons) in a given sample] for carbon and nitrogen isotopes demonstrated the feasibility of distinguishing fish fed FM-free diets (Fig. 4); these expressed lower levels of the nitrogen isotope δ15N compared against the FMC and COM fed fish. Levels of the carbon isotope δ13C varied, with values for COM fed fish being greater than recorded in other diets.
On the organoleptic evaluation, of the 25 participants 12 stated – based on taste, texture and aroma – a preference for the FFF fed fish, three indicated no preference and 10 preferred fish raised on the COM diet.
Perspectives
Results from the trials described above unambiguously demonstrate that marine ingredients can be totally withdrawn from largemouth bass dietary formulations without negatively influencing fish growth, health, or consumer acceptance. The trials thereby support the recent viewpoint of an Advocate article that it is not feed ingredients that matter, but the balance of nutrients that are of greatest importance.
Nevertheless, even though the current trial series was successful, there remains a need to continue to develop and fine-tune diets that incorporate a fusion of terrestrial proteins and oils that work synergistically to the benefit of animal performance, welfare, product safety and quality and, where needs be, processability.
Commercial dietary formulations already include a wide variety of plant and animal proteins and oils, supplemented with pre- and probiotics, essential amino acids, vitamins, minerals and other constituents. An important facet of the presented trials was the application of stable isotope ratio mass spectrometry (SIRMS) to authenticate that fish fillets were derived from fishmeal and fish oil-free fed animals and this proved successful. The study confirms and extends the findings with salmon, shrimp, and other species (DOI: 10.1007/s00217-014-2298-5; 10.1016/j.foodcont.2015.01.003) and thereby supports the use of SIRMS for verification purposes of the presence of marine ingredients. Other methods for establishing the origin and authenticity of aquacultured products are nonetheless deserved of evaluation (DOI: 10.1016/j.tifs.2019.07.010).
It has been suggested that alternative proteins, and especially those of vegetable origin, influence textural and organoleptic qualities of fillets. These suspicions are supported by measured changes in fillet composition, and especially that of fat, deviations in muscle cell/fiber size, mechanical evaluations of fillet texture and gene expression analyses (DOI: 10.1016/j.aquaculture.2004.01.006; 10.111/jwas.12452; 10.1016/j.aquaculture.2006.12.012; 10.1016/j.aquaculture.2015.11.034).
These findings are of critical importance since alterations in fillet quality characteristics may influence not only consumer acceptance and fish processing, but market demand. Herein, fishmeal and fish oil replacement had no discernable impact of the eating quality of largemouth bass. Similar findings have been made for a broad variety of species including, but not limited to, blunt snout bream and gilthead sea bream (DOI: 10.1111/anu.1258110; 1016/j.aquaculture.2012.07.009) and, using various fishmeal replacers, rainbow trout (DOI: 10.1016/0044-8486(94)00403-B; 10.1111/j.1749-7345.2010.00441.x; 10.1046/j.1365-2095.1998.00077.x). Upcoming studies, with the objectives of the F3 in mind, have already been planned using other carnivorous species that rely heavily on fishmeal and fish oil inputs.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Ewen McLean, Ph.D.
Corresponding author
Aqua Cognoscenti LLC, West Columbia, South Carolina, USA[109,111,99,46,108,105,97,109,103,64,110,97,101,108,99,109,46,110,101,119,101]
-
Luke Fredriksen
Aquatic Research Laboratory
Prairie Aquatech
Brookings, South Dakota, USA -
Kelly Alfrey
Anthropocene Institute
Palo Alto, California, USA -
Michael F. Tlusty, Ph.D.
School for the Environment
University of Massachusetts Boston
Boston, USA -
Steven R. Craig, Ph.D.
Optimal Fish Food, Brookings
South Dakota, USA -
Frederick Barrows, Ph.D.
Aquatic Feed Technologies LLC
Bozeman, Montana, USA
Tagged With
Related Posts

Aquafeeds
Counterpoint: Marine ingredients are stable in volume, strategic in aquaculture nutrition
IFFO Director General Petter M. Johannessen says fishmeal and fish oil offer unmatched nutrition and benefits to fuel aquaculture’s growth trajectory.

Aquafeeds
Aquaculture Exchange: Rick Barrows
After 14 years with the USDA’s Agricultural Research Service, Rick Barrows talks about the importance of finding ‘complete’ and commercially viable alternative sources of omega-3 fatty acids and continuing innovation in the aquafeed sector.

Innovation & Investment
Talking innovation in the cradle of aquaculture
This year’s GOAL conference will be held in China, birthplace of aquaculture. There’s no better place to discuss innovation, says Michael Tlusty, director of ocean sustainability science at the New England Aquarium, because fresh thinking defeats complacency.

Aquafeeds
Study replaces dietary fish oil with microalgal oil
Study compares the response of Pacific white shrimp fed microalgal oil-based diets and assessed their ability to withstand a Vibrio parahaemolyticus challenge.


