Bacterial community useful in start-up of a system, after disinfection following a disease outbreak

The use of biofloc technology for super-intensive shrimp production is emerging as a sustainable alternative to traditional shrimp culture. The advantages of super-intensive biofloc production include temperature control for year-round production, the ability to minimize the introduction and spread of shrimp pathogens, and drastic reductions in water use and effluent discharge. Biofloc management relies on in situ microbes, including bacteria and algae, to prevent the accumulation of the inorganic nitrogenous metabolites ammonia and nitrite, which can be toxic to shrimp.
A heterotrophic bacterial community can minimize ammonia accumulation through assimilation into bacterial biomass. This bacterial community can be promoted by manipulating carbon:nitrogen ratios in the water column through the use of low-protein feeds and the addition of an organic carbon source such as molasses.
A chemoautotrophic bacterial community (i.e., nitrifying bacteria) can prevent the accumulation of ammonia and nitrite through oxidation to nitrate. This bacterial community can be maintained by replacing alkalinity consumed during nitrification with an inorganic carbon source such as sodium bicarbonate or calcium carbonate.
Depending upon light availability and management strategy, photoautotrophs (i.e., microalgae) can also be present. They minimize ammonia and nitrate accumulation through assimilation into algal biomass.
Heterotrophic bacteria have short generation times and can be established quickly, whereas nitrifying bacteria grow and divide more slowly and require careful management in a biofloc system. Biofloc strategies for super-intensive shrimp production typically include members of all three of these microbial communities.
Establishing nitrifying bacteria
Experienced home aquarists understand that “aged” biofilter media or gravel, which has an established nitrifying bacterial community, is helpful when starting a new aquarium. If such media are not available, the aquarium can be allowed to “cycle” naturally by maintaining an appropriate number and type of fish until cycling is completed.
Fish used during cycling excrete ammonia, which serves as a substrate for ammonia-oxidizing bacteria (AOB). Ammonia is oxidized by the bacteria to nitrite, which in turn provides substrate for nitrite-oxidizing bacteria (NOB). Nitrite is oxidized by NOB to relatively non-toxic nitrate. In these steps, ammonia or nitrite must accumulate in the water before the respective nitrifying bacterial groups become established.
This cycling process, which can take four to six weeks, is characterized by spikes in ammonia and nitrite concentrations as AOB and NOB become established. Several strategies can accelerate the cycling process and minimize the spikes.
One such strategy is to maintain a minimal level of ammonia and/or nitrite at the start of the cycle through the chemical addition of ammonium chloride and/or sodium nitrite. This provides substrate for nitrifying bacteria immediately, rather than waiting for ammonia and nitrite to accumulate naturally. If cycling is not conducted properly in a home aquarium, “new tank syndrome,” where ammonia and/or nitrite can increase to lethal levels, can result.
The same concepts apply to the start-up of biofloc systems used for super-intensive shrimp production. Importantly, issues associated with improper cycling are exacerbated by super-intensive stocking densities and rapid shrimp growth. Feed rates and subsequent ammonia and nitrite production are high, and with insufficient cycling, this can result in much faster and higher spikes of ammonia and/or nitrite that can have sub-lethal or lethal effects on the shrimp.

Super-intensive biofloc system
Research on super-intensive shrimp culture with biofloc has been conducted at Oceanic Institute in Waimanalo, Hawaii, USA, since 1997. The institute maintains a selective-breeding program for Pacific white shrimp, Litopenaeus vannamei, which requires growout evaluation of selected families. These trials are conducted in a 75-cubic-meter super-intensive BFT raceway stocked at 300-400 shrimp per cubic meter in Oceanic Institute’s Nucleus Breeding Center.
Strict biosecurity protocols in the breeding center protect the valuable stocks from potential pathogens. The raceways are chlorinated and rinsed with freshwater between trials. Each new super-intensive trial is started with clear seawater.
Difficulties in establishing adequate nitrifying bacterial communities during the cycling periods of several past trials resulted in nitrite spikes that sometimes exceeded 25 mg/L nitrite-nitrogen. Such elevated nitrite concentrations resulted in poor shrimp health and at times has required flushing of the system.
Better control of the spikes in nitrite during cycling was needed. Techniques such as the addition of biofilter backwash, nursery system water and starter nitrifying bacterial cultures resulted in limited success. In addition, these resources were not consistent in quality and availability, or were costly.
Sodium nitrite addition
Nitrite-oxidizing bacteria have a doubling time of at least 15 hours, whereas ammonia-oxidizing bacteria can double in about half that time. The shorter generation time likely explains the observation that ammonia cycling spikes in Oceanic Institute’s super-intensive biofloc system never reached toxic levels, and AOB became established more easily than NOB.
Because nitrite toxicity was a primary concern, experiments with the addition of nitrite in the form of sodium nitrite were conducted. The addition of sodium nitrite prior to stocking shrimp would provide the substrate necessary to allow naturally occurring NOB to become established immediately. Importantly, nitrifying bacteria are sensitive to ultraviolet radiation when unattached, so black shade cloths were installed over the raceway during cycling.
Fig. 1 shows nitrite-nitrogen concentrations in two trials in which multiple doses of sodium nitrite were added (trials 4 and 5) and prior trials where nothing was added (trial 1). In trial 2, biofilter backflush and one dose of sodium nitrite were added. Only biofilter backflush was added in trial 3.
During trial 5, the biofloc raceway was filled two weeks prior to stocking and initially spiked with 1-2 mg/L of sodium nitrite-nitrogen. Five subsequent doses were added to the raceway in an attempt to maintain a minimal nitrite concentration. Fig. 2 shows total ammonia-nitrogen, nitrite-nitrogen and nitrate-nitrogen prior to stocking and during the cycling period of trial 5, which was stocked at a density of 300 shrimp per cubic meter.
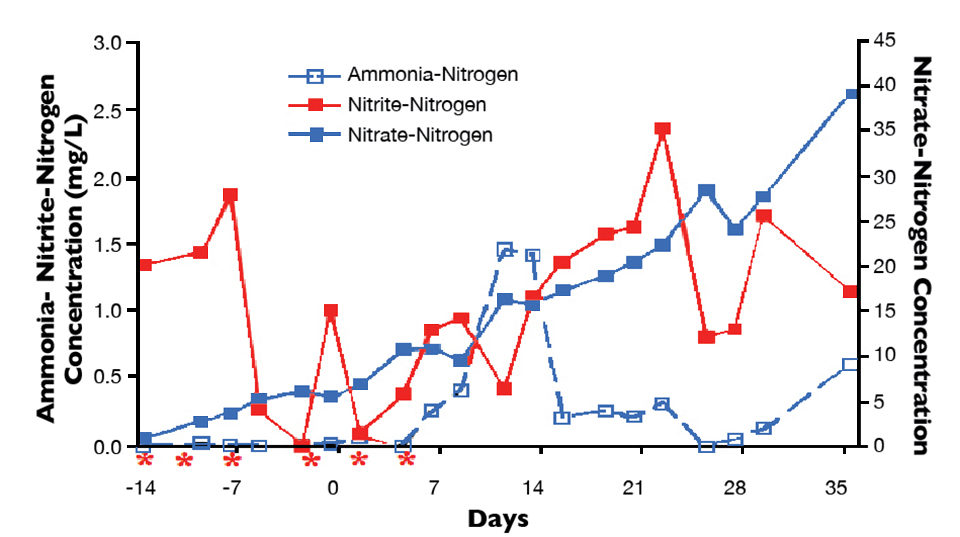
The increase in nitrate-nitrogen just days after sodium nitrite was first added confirmed the establishment of NOB by demonstrating that nitrite was being oxidized to nitrate. The presence of NOB early in the cycling period presumably allowed this group of bacteria to react more quickly to the natural accumulation of nitrite after shrimp were stocked.
No significant spikes in nitrite concentration were observed during or subsequent to the cycling period of trial 5. Importantly, expensive nitrifying bacteria starter cultures were not needed, as there were sufficient nitrifiers in the incoming seawater to develop a community rapidly when provided with an appropriate substrate.
Perspectives
There are many scenarios where a simple, cost-effective protocol to establish a nitrifying bacterial community would be useful, such as initial start-up of a system, start-up after disinfection following a disease outbreak, lack of aged water, the desire to limit cross-contamination with aged water or the start-up of intermittent research trials.
During trial 5, 4.3 kg of sodium nitrite were used during the cycling period for the 75-cubic-meter raceway at a cost of $10.63. Data from trial 5 suggested that a shorter start-up period and less sodium nitrite dosing may be adequate to complete proper cycling. Future investigations will focus on optimizing the timing and amount of sodium nitrite dosage in an effort to further reduce cost and labor.
(Editor’s Note: This article was originally published in the May/June 2011 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Clete A. Otoshi
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA[103,114,111,46,101,116,117,116,105,116,115,110,105,99,105,110,97,101,99,111,64,105,104,115,111,116,111,99]
-
Neil Rodriguez
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA -
Shaun M. Moss, Ph.D.
Oceanic Institute
41-202 Kalanianaole Highway
Waimanalo, Hawaii 96795 USA
Tagged With
Related Posts

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Intelligence
A land grab for salmon (and shrimp) in upstate New York
The operators of Hudson Valley Fish Farm see their inland locale as a pilot to prove that land-based fish farming, located in close proximity to major metropolitan markets, can be successful.

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.

Health & Welfare
Biofloc trial results in fast shrimp growth, low FCR, high survival
A trial in a lined, greenhouse-enclosed raceway evaluated the use of a heterotrophic biofloc system equipped with aeration, supplemental oxygen injection and centralized heating to achieve good shrimp production.



