Bacillus species capable of highest growth with syrup substrate

Since the syrup contains organic carbon sources, microbes have also been used to convert it into other desirable products. However, the syrup remains an underutilized component of the ethanol production process. In this study, the syrup was used as a nutrient-rich medium for microbial biomass development, which, if successful, would improve the profitability of ethanol production by developing a possible new protein source for aquaculture feeds.
Large-scale cultivation of microbial biomass has been used in a variety of industrial practices, including production of agricultural probiotics, carotenoid production, human and agricultural food production, and wastewater treatment. The aquaculture industry has a particular interest in culturing protein-rich bacterial biomass on wastewater/byproducts to replace fishmeal in aquaculture feeds. Fishmeal has traditionally served as the major protein source for many cultured species of fish and shellfish due to its high palatability and digestibility and its well-balanced essential amino acid profile. However, fishmeal demand has increased along with the price, which makes alternative protein sources more lucrative to the aquaculture industry.
Culturing microbial biomass on wastewater/byproducts has the added benefit of making use of these otherwise no- to low-value waste streams. It has been previously demonstrated that microbial biomass can be cultured while treating wastewater from fish farms and confectionary manufacturing plants and this biomass can be successfully used to replace fishmeal in feeds for shrimp grown in aquaculture.
This article – adapted and summarized from the original publication – investigated the capacity for microbes to grow on a corn ethanol fermentation syrup substrate so that they might also be used as a protein supplement in aquaculture feeds. The study findings lay the groundwork for future application of the bacterial biomass in aquaculture.
We thank Brian Badgley, Silke Hauf, Christopher Lawrence and Stephen Melville at Virginia Tech for sharing procedures and equipment and Jason Bootsma for serving as our liaison with Flint Hills Resources.
Study setup
A bioreactor-grown soil enrichment community capable of metabolizing the syrup was first established and its community profile was characterized at a molecular level using 16S rRNA sequences. Then, defined monocultures were tested for their growth yields, and binary culture combinations were examined for possible synergistic effects within the community. Bacillus species, although not the dominant organism in the bioreactor, were the most productive pure culture isolates.
Syrup, a byproduct of ethanol production, was obtained for use as a growth substrate for bacteria. To examine how robust the bacterial growth would be across different lots of syrup, three separate batches were provided from three different ethanol production facilities with similar design and function. The three syrups were removed at the same point in the ethanol production process, after oil separation and evaporation through the application of centrifugation and high temperature (~85 degrees-C), respectively.
Although three different commercial yeast strains were used, the CCDS produced were all similar in content with ~30 to 40 percent solids and ~60 to 70 percent water. Syrup 2 was used for all studies, while syrups 1 and 3 were examined in monoculture studies to test for organism robustness.
For additional information on the syrup growth substrate; temporal community profiling of bacterial enrichment in anaerobic reactors; MiSeq data analysis; Pure culture strain isolation from enrichment cultures; monoculture and binary-combination growth assays of microbial strains; strain identification; and accession numbers, please consult the original publication.
Results and discussion
The purpose of this study was to identify pure culture isolates obtained from laboratory stocks and a soil enrichment community that could grow using a byproduct of ethanol fermentation production as their sole nutrient source. At the final time point (day eight) of the anaerobic digestion with the syrup and soil (Fig. 1), there were seven dominant organism families that were enriched including the Bacillaceae family that proved to be the group of organisms of greatest interest. The other six families enriched in the bioreactor were Clostridiaceae, Alicyclobacillaceae, Ruminococcaceae, Burkholderiaceae, Veillonellaceae and Enterobacteriaceae. However, of those enriched, only four, the Bacillaceae, Burkholderiaceae, Alicyclobacillaceae, and Enterobacteriaceae families, have members that are facultative anaerobes.
Our procedures were designed to select against organisms that were obligate aerobes or anaerobes. Facultative growth is an ideal trait that enables handling of organisms under aerobic conditions, while allowing fermentation in large-scale industrial vats.
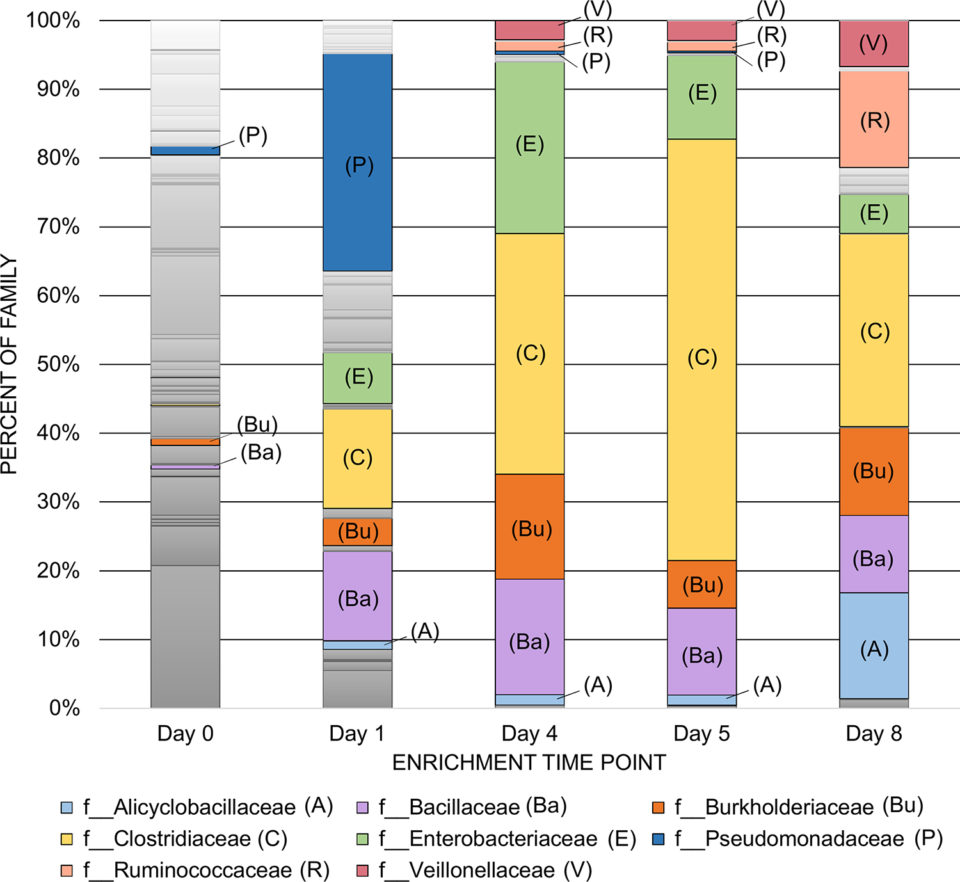
The monoculture assays revealed Bacillus species as the organisms capable of the highest levels of growth with the syrup substrate. While the most productive monocultures exclusively belonged to the Bacillaceae family, the enrichment study revealed a more diverse community of seven dominant families capable of growth on the syrup. In fact, the Bacillaceae family represented just 11.3 percent of the total community. Antagonism and/or competition were probable contributing factors within the reactors limiting the growth of the Bacillaceae. However, the Bacillaceae were the most successful group of organisms at adapting to the different selections we applied with regard to oxygen availability (i.e. anaerobic and aerobic growth) and medium choice (TSA and syrup). Since variability in syrup composition and nutritional content is known, a highly desirable trait is the capacity for robust growth across different batches. The Bacillaceae examined appear to have this desired characteristic.
Interestingly, numerous studies have shown Bacillus sp. to be able to utilize multiple carbon sources, and they are capable of catabolite repression when grown in the presence of more than one source. More recently, interest in Bacillus sp. metabolism has increased regarding carbon sources that are industrially relevant. Since the process used by the production facilities does not entail very high temperatures, with all steps occurring at less than 87.8 degrees-C, it would be unlikely that anhydro sugars would be formed. Instead, glycerol, dextrin (DP4), and maltose (DP2) were the most abundant carbohydrates in the solid fraction of the syrups.
Thirty-four binary combinations of the microbes were grown on the syrup to see if the initial syrup substrate might be interconverted into metabolites better supporting growth of the mixed community. The binary growth assays revealed no apparent synergistic growth effects, as none of the combinations tested grew any better than did just one of the individual organisms. This indicates that there are no beneficial metabolites produced for the paired organisms to use. Despite this, synergism could be possible between the top Bacillus species isolates and other organisms that were present in the initial bioreactor community.
The discovery that Bacillus species utilize the syrup as their sole nutrient source has the potential for future applications. For example, bacteria in the Bacillus genus have been used as a supplement with aquaculture feed in industrial practices, specifically for probiotic benefit, stimulating fish immune system, and even improving water quality. In addition, their ability to form highly stable dormant spores makes long-term transport and storage possible. By dry weight, Bacillus cells are roughly 50 percent protein, thus they could be used as a supplement instead of expensive fishmeal in the feed of aquaculture-grown animals. This study identified some promising Bacillus isolates that would be considered safe for animal consumption as a substitute for fishmeal.
Perspectives
Fishmeal supply trends suggest a need for additional and alternative nutritional material for permanent aquafeed supplement. The use of the syrup substrate as a means to cultivate microbes, such as Bacillus species, could provide enhanced economic and sustainability benefits not only to the process of ethanol production, but also to commercial aquaculture.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Holly Packard
Department of Biological Sciences
Virginia Tech
Blacksburg, VA, United States of America -
Zachary W. Taylor
Department of Biological Sciences
Virginia Tech
Blacksburg, VA, United States of America -
Stephanie L. Williams
Department of Biological Sciences
Virginia Tech
Blacksburg, VA, United States of America -
Pedro Ivo Guimarães
Department of Biological Systems Engineering
Virginia Tech
Blacksburg, VA, United States of America -
Jackson Toth
Department of Biological Systems Engineering
Virginia Tech
Blacksburg, VA, United States of America -
Roderick V. Jensen, Ph.D.
Department of Biological Sciences
Virginia Tech
Blacksburg, VA, United States of America -
Ryan S. Senger, Ph.D.
Department of Biological Systems Engineering and Department of Chemical Engineering
Virginia Tech
Blacksburg, VA, United States of America -
David D. Kuhn, Ph.D.
Department of Food Science and Technology
Virginia Tech
Blacksburg, VA, United States of America -
Ann M. Stevens, Ph.D.
Corresponding author
Department of Biological Sciences
Virginia Tech
Blacksburg, VA, United States of America
Tagged With
Related Posts

Intelligence
Byproduct utilization for increased profitability, part 1
Protease enzymes are important industrial enzymes that have diverse applications in food, leather, silk and the agrichemical and pharmaceutical industries. Fish are considered one of the richest sources of proteolytic enzymes.

Responsibility
Bacterial amendments in shrimp grow-out ponds
Pond microbial communities are a critical and often overlooked component of aquaculture ecosystems. Bacterial amendments like probiotics provide significant support to shrimp farmers around the world.

Responsibility
Integrated utilization of microalgae grown in aquaculture wastewater
A study shows that wastewater from recirculating aquaculture systems is suitable for microalgal cultivation and that sludge amendment to the wastewater increases production.

Responsibility
Aquaculture byproducts improve sustainability of seafood value chains
Tons of aquaculture byproducts are available as sources for fishmeal and fish oil to supplement the supplies obtained from fisheries. Innovative technologies are supporting more efficient use of these by-products in aquafeed.


