Resulting product reached adequate levels of protein and lipid concentrations

Many byproducts coming from the seafood industry – such as viscera, skin, scales and bones, representing up to 30 to 80 percent of the fish body weight – are discarded as solid wastes by industrial fish-processing operations. However, due to their composition, they have great potential to be used as protein supplements in aquaculture feeds.
Their conversion to ingredients is also encouraged by their significant advantage in that they do not require any thermal-chemical and/or enzymatic hydrolysis pretreatment steps. Since the pretreatment step is neither economically favorable nor environmentally friendly, its elimination from the process makes the utilization of fish waste economical and more environmentally friendly.
Biotechnological methods like fermentation with microbe cultures are becoming more popular for the treatment of waste. Among the microorganisms applied, yeasts have also been used as an inoculum, along with lactic bacteria, to ferment fish waste for converting it to a useful product that can be used as an ingredient to balance the food rations of animals. Yeast has many different immunostimulatory compounds, including nucleic acid, beta-glucans and mannan oligosaccharides. These compounds may enhance the growth of different fish species and can therefore be considered as excellent health promoters for cultured aquatic species.
Fermented fish waste is a liquid product, obtained by the liquefaction of tissues carried out by the enzymes already present in the fish and accelerated by an acid pH. Natural fillers, such as agricultural byproducts, can also be added to the substrate. For example, citrus peel can be used as a filler during fermentation, playing at the same time an important role as a prebiotic source. Among its beneficial effects, it has been reported that prebiotics can elevate fish resistance to pathogens and improve growth performance, feed utilization and lipid metabolism, as well as stimulate the immune response through the modulation of intestinal microbiota.
This article – summarized from the original publication (Tropea, A. et al. 2021. Aquafeed Production from Fermented Fish Waste and Lemon Peel. Fermentation 2021, 7(4), 272) – presents the results of a study to process non-sterilized fish wastes from non-edible parts of processed European seabass (Dicentrarchus labrax), supplemented with lemon peel as a filler and prebiotic sources, through biological fermentation using combined starter cultures of the yeast Saccharomyces cerevisiae and the probiotic bacterium Lactobacillus reuteri for bio-transforming these byproducts into an aquafeed supplement with high protein content and rich in healthy microorganisms.
Study setup
Non-edible byproducts of European sea bass (heads, viscera, skin and bones) were provided by Acqua Azzurra S.p.a. (Pachino, Italy). Samples were collected directly at the farm and forwarded to the laboratory under refrigerated conditions. Lemon peel was provided by Simone Gatto S.r.l. (San Pier Niceto, Italy). Samples were stored at minus-20 degrees-C until tests were performed. Microorganism, yeast and probiotic bacterium samples were also procured from commercial sources.
Fermentation tests were carried out in a commercial, 5-liter batch fermenter. Fish waste and lemon peel (2:1 weight/weight) were homogenized in a blender for 5 minutes and supplemented with 20 mL of S. cerevisiae (108 cells per mL) and 20 mL of L. reuteri culture (108 cells per mL). No sterilization procedures were used. All fermentations were carried out for 120 hours until no further growth of the selected microorganisms was observed, and the pH value became stable. The pH was not controlled by alkali addition during cultivation. Medium samples were withdrawn daily from the reaction vessel using a sterile 20 mL syringe and immediately frozen at minus-20 degrees-C until analysis.
For detailed information on the experimental design, fermentation and analytical and statistical procedures, consult the original publication.
Upcycling food system byproducts in livestock and aquaculture feeds
Results and discussion
Regarding substrate fermentation, the growth of S. cerevisiae was slow during the first 24 hours of fermentation, maintaining a concentration of 108 CFU/g. S. cerevisiae reached a concentration of 1011 CFU/gram after 72 hours, remaining stable until the end of the process. L. reuteri increased constantly from the beginning of the fermentation until after 96 hours, increasing in concentration from 108 up to 1012 CFU/gram, and reaching a steady state until the end of the process.
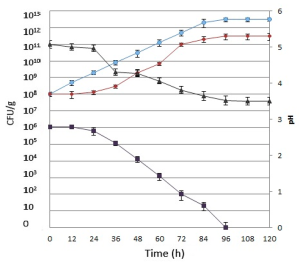
The reduction in pH was slow during the first 24 hours of fermentation because of expected microorganism adaptations at the beginning of the process. In the presence of lactic acid bacteria (LAB) and yeast, after 24 hours the pH of the mixture became stable at 3.5. The decrease in pH in the substrate offers evidence of good acidification through lactic fermentation by the starter cultures and represents the most important factor to control in biotransformation.
Acidification must be achieved as quickly as possible, to inhibit the growth of pathogenic and spoilage microorganisms in the substrate and increase the shelf life of the resulting fermented substrate. Moreover, considering that no sterilization procedures were done, the quick drop in pH was found to be necessary for maintaining microbial hygiene and for retaining the quality of the product as an aquaculture feed. In fact, the amount of initial substrate total coliforms was 106 CFU/gram, but no fecal coliforms were detected. The microbiological analysis for total coliform determination showed a net decrease during the fermentation, to reach a complete absence after 96 hours (Fig. 1).
The reduction in coliform numbers could be due to some inhibitory compounds (bacteriocins) formed by the microorganisms employed during lactic acid fermentation and/or to the acidification of the medium. Moreover, the decrease in coliforms may ensure good biopreservation against undesirable and/or hazardous microorganisms. The final fermented products were low in spoilage microorganisms and rich in healthy microorganisms, representing a healthy final substrate enriched with added value.
The capability of the starter cultures to grow at low pH can be ascribed to the lemon peel supplementation since polysaccharides (such as pectins) show a protective effect on lactic acid bacteria (LAB) against low pH. Their ability to achieve this on fermenting fish waste supplemented by lemon peel was confirmed by the protein levels increasing during the process, to up to 48.55 percent, making these wastes an excellent raw material for aquafeed production with added Lactobacillus reuteri and Saccharomyces cerevisiae.
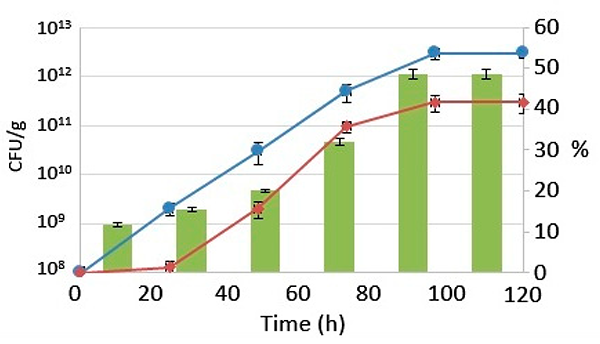
The substrate’s initial protein content was 11.68 ± 0.48 percent. It increased slowly after 72 hours, reaching up to 32.09 ± 0.77 percent. The highest protein percentage in the substrate, 48.55 ± 1.15 percent, was reached after 96 hours. This value remained stable until the end of the fermentative process, producing a substrate rich in protein and achieving a suitable level for aquafeed formulation. Fig. 2 shows the protein content against the CFU of the yeast and lactic acid bacteria (LAB).
During all fermentations, ash concentrations decreased significantly, from 0.83 ± 0.04 percent to 0.66 ± 0.03 percent. This could be due to partial ash utilization by the yeast as a source of minerals. The crude lipid content calculated on the initial substrate was 13.74 ± 0.72 percent. Throughout the process, this value did not increase significantly; at the end of the fermentation period, it reached just 15.25 ± 0.80 percent.
Concerning the content of fatty acids at different fermentation times, the main ones detected were palmitic, oleic and linoleic acids. The concentration of saturated fatty acids was not affected by the fermentation process, whereas monounsaturated and polyunsaturated ones showed an opposite trend, increasing and decreasing, respectively, during the process.
Perspectives
This study demonstrated an effective approach to utilizing seafood processing waste as a fermented substrate for aquafeed ingredients and nutrients, starting with fish and lemon peel wastes, and converting the animal and vegetable food wastes into an added-value product. The final fermented product of our study is low in spoilage microorganisms and rich in healthy microorganisms, representing a healthy final substrate enriched with added value.
The microorganisms’ ability to feed on fermenting fish waste that is supplemented by lemon peel was confirmed by the protein levels increasing during the process, to up to 48.55 percent, making these wastes an excellent raw material for aquafeed production via the addition of L. reuteri and S. cerevisiae. The final protein and lipid contents represent adequate levels as aquafeed ingredients.
Further studies are in progress for converting the resulting fermentation product into pellets and for testing the effect of the final product on the growth and immune response of cultured fish. Additional work will be needed to further optimize production to facilitate future larger-scale production, also evaluating it from an economic point of view.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Alessia Tropea
Corresponding author
BioMorf Department, University of Messina, 98168 Messina, Italy[116,105,46,101,109,105,110,117,64,97,101,112,111,114,116,97]
Tagged With
Related Posts

Aquafeeds
Calysseo launches industrial-scale facility to produce microbial protein for the aquaculture market
Calysseo has launched an industrial-scale facility in China to produce FeedKind® – a microbial protein for the aquaculture market.
Aquafeeds
Canada’s CFIA approves KnipBio Meal as a salmon feed ingredient
The Canadian government has approved a single-cell protein from U.S. biotechnology company KnipBio for inclusion in farmed salmon feeds.

Innovation & Investment
Innovation Award 2020 finalist: ME-PRO® from Prairie AquaTech
An alternative aquaculture feed ingredient made in the American Plains is a finalist for GAA’s annual Global Aquaculture Innovation Award.

Aquafeeds
Testing alternative diet compositions to offer more sustainable feed formulations for European sea bass
Alternative diets using novel plant and animal-derived protein sources have comparable performance to current commercial sea bass feeds.



