Performance evaluated at the University of Arkansas-Pine Bluff

Recirculating aquaculture systems (RAS) are gaining interest due to their decreased requirements for land and water, and their ability to be located close to markets. One important water treatment process in RAS is biofiltration. As fish metabolize the protein in feed, ammonia is produced as a waste product. Unionized ammonia, the proportion of which is determined by temperature and pH, is highly toxic to fish.
Biofilter media provides surface area for the growth of Nitrosomonas bacteria that convert ammonia to nitrite, and Nitrobacter bacteria that convert nitrite to nitrate. Bead filters are designed to function as a physical filtration device to remove solids while simultaneously encouraging the growth of these desirable bacteria. As solids accumulate in the bead filter, they can limit the nitrifying bacteria’s access to chemicals (e.g, oxygen, ammonia, and nitrite) in the water and impede nitrification.
To enhance biofilter nitrification performance, a settling chamber can be placed inline to reduce solids accumulation in the filter bed. The filter performance of a bubble-washed bead filter with partial solids removal was evaluated in an RAS for growing tilapia in a greenhouse at the Agriculture Experiment Station of the University of Arkansas in Pine Bluff, Arkansas, USA.

System description
The system (Fig. 1) consisted of six 1-meter-diameter polyvinyl tanks containing 540 liters of water each. Water gravity-drained from the tanks into a 5-cm-diameter manifold that emptied into a rectangular 1,200-liter settling tank with a curved bottom.
The first of two 1.2-cm chambers in the settling tank (water depth 0.7 meters) served as a sedimentation basin. Water overflowed a weir from this chamber into the second chamber (water depth 0.5 meters), which served as the sump basin. Water from the sump basin was pumped into the bubble-washed bead filter containing 0.06 cubic meters of modified polyethylene bead media. Water outflow from the bead filter was divided evenly among the six culture tanks.
An 1,800-watt heater was placed in the first chamber of the settling tank to maintain system water temperature at 25 degrees-C. A 15-cm air stone was placed in each tank for aeration supplied by a 65-watt piston compressor. Sodium bicarbonate was added as needed to maintain alkalinity levels above 100 milligrams per liter. The bead filter was backflushed each morning to remove captured solids. The culture tanks were stocked with 3.8-kg fish at 100 fish per tank. The fish were fed a 32 percent-protein floating catfish feed.
Evaluating filter performance

Bead filter performance was evaluated at feeding rates of 450, 675 and 900 grams per day. During the evaluation periods, samples were collected for four consecutive days at 0, 6, 12 and 24 hours after the filter was backflushed. The fish were fed once per day at the lowest feeding rate and twice per day for the two higher feeding rates.
Filter evaluation began two weeks after the feeding rate was increased to allow the system to reach steady state. Measured system parameters included temperature, pH, dissolved oxygen (DO), total ammonia nitrogen (TAN), and nitrite nitrogen. For filter evaluation TAN, nitrite and DO samples were collected from the filter inflow and outflow ports.
Filter performance was evaluated by calculating volumetric TAN conversion rate (VTR) using the formula:
VTR = Kc (TANI – TANE) Qr/Vb
VTR = volumetric TAN conversion rate
(g TAN converted/m3 filter media per day)
Kc = unit conversion factor of 1.44
TANI = influent ammonia concentration (mg N/l)
TANE = effluent ammonia concentration (mg N/l)
Qr = flow rate through the filter (lpm)
Vb = total volume of bead media (cubic meters).
Test results
Physical and chemical water quality mean values for each feeding rate are presented in Table 1. Bead filter outflow dissolved oxygen should be above 2 milligrams per liter to prevent the nitrifying bacteria from being oxygen-limited.
Lenger, Physical and chemical water quality values, Table 1
| Feed Rate (g/day) | Temp (°C) | Tank pH | DO (mg/l) | TAN (mg/l) | NO2 (mg/l) | Qr (lam) |
|---|---|---|---|---|---|---|
| 450 | 24.8±2.1 | 7.4±0.1 | 5.8±0.9 | 0.56±0.09 | 0.25±0.07 | 79±0.03 |
| 675 | 27.0±3.0 | 7.9±0.2 | 5.0±1.0 | 0.54±0.10 | 0.10±0.04 | 57±0.06 |
| 900 | 26.7±1.4 | 7.6±0.1 | 4.9±0.7 | 0.84±0.15 | 0.18±0.14 | 40±0.10 |
At the lowest feeding rate, aeration in each culture tank was sufficient for maintaining the bead filter outflow oxygen concentration above 2 milligrams per liter. However, at the higher feeding rates, it was necessary to place two additional air stones in the lower chamber of the settling basin to maintain outflow oxygen concentration greater than 2 milligrams per liter.
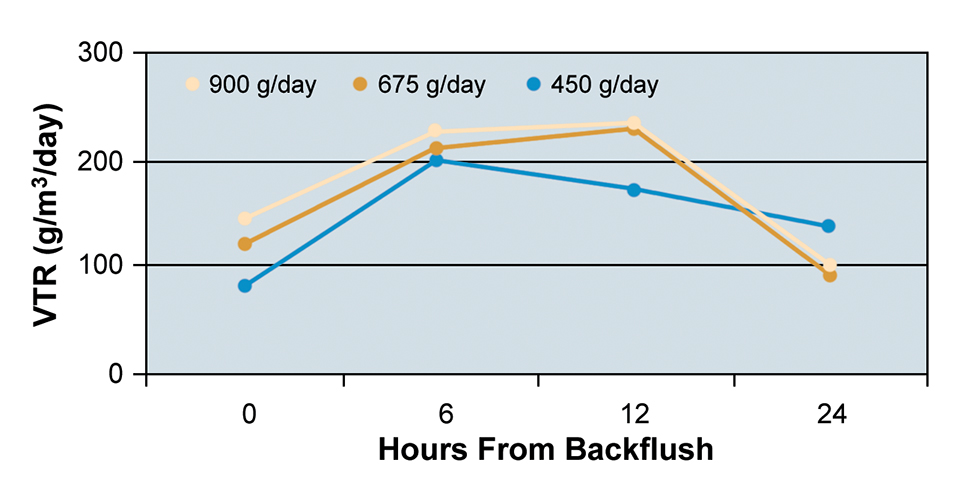
At the three feeding rates tested, the bead filter was able to maintain TAN and nitrite concentrations at acceptable levels for tilapia culture. Mean VTR values for each feeding rate at each sampling period are presented in Fig. 2.
An increase in VTR was observed six hours after backflushing at each of the three feeding rates due to decreased solids in the bead filter and consequently increased chemical diffusion within the biofilm. VTR dropped after six hours at the feeding rate of 450 grams per day and after 12 hours at the rates of 675 and 900 grams per day. The decrease in VTR was mainly a result of solids accumulation in the filter bed and reduction in flow through the filter.
At the 900 grams per day feeding rate, the system TAN was highest despite the high VTR. This was due to the increased loading of the system and the decreased tank turnover time. At the two higher feeding rates, solids accumulated and clogged the drain manifold. This decrease in flow through the manifold required decreasing the flow through the bead filter.
Flow to the filter was decreased to prevent overdrawing the sump basin and consequently letting air enter the bead filter, disrupting the static filter bed and allowing captured solids to reenter the system. A larger-diameter manifold should have been used to feed the settling basin. As a result, water flow through the filter would have increased and system TAN levels at the highest feed rate would be lower.
Conclusion
The bead filter was able to maintain TAN and nitrite at acceptable concentrations for RAS tilapia culture, despite operating at only 66 percent of design performance criteria. Increasing the backflush frequency, improving prefilter solids capture, or increasing the bead filter size can accommodate the higher ammonia levels observed at higher feeding rates.
Removal of solids by prefiltration or increasing backflush frequency enhances filter nitrification by removing solids that encourage the growth of heterotrophic bacteria which produce ammonia and compete with nitrifying bacteria for oxygen and space. Additionally, captured solids can cause channeling of water through a biofilter, thereby minimizing its nitrification abilities. Backflushing also removes excess biofloc and ensures a thin biofilm that promotes the growth of nitrifying bacteria.
Monitoring changes in VTR is a valuable management tool that can reveal when filters require backflushing to maintain optimum nitrification rates and consequently maintain the low system TAN levels required for fish culture. Increasing backflush frequency and using a prefilter for partial solids capture are two methods of maintaining high VTR in bead filters.
(Editor’s Note: This article was originally published in the June 2001 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Todd A. Lenger
Aquaculture/Fisheries Center
University of Arkansas at Pine Bluff
Pine Bluff, Arkansas, USA tlenger@uaex.edu[117,100,101,46,120,101,97,117,64,114,101,103,110,101,108,116]
-
Peter W. Perschbacher, Ph.D.
Aquaculture/Fisheries Center
University of Arkansas at Pine Bluff
Pine Bluff, Arkansas, USA tlenger@uaex.edu[117,100,101,46,120,101,97,117,64,114,101,104,99,97,98,104,99,115,114,101,112,112]
-
Timothy J. Pfeiffer, Ph.D.
USDA/ARS Aquaculture Systems
Research Unit
Pine Bluff, Arkansas, USA[117,100,101,46,120,101,97,117,64,114,101,102,102,105,101,102,112,116]
Related Posts

Health & Welfare
Ammonia addition enhances microbial flocs in nursery phase for Pacific white shrimp
In a study, “pre-fertilization” in the nursery phase of a biofloc system for shrimp was tested. The objective was to accelerate the biofloc formation to minimize ammonia concentrations, avoiding high peaks during culture.

Responsibility
Denitrification in recirculating aquaculture systems: Comparison of aquatic plants
Denitrification by anaerobic bacteria can remove nitrates from RAS, but the operation requires anaerobic conditions and the addition of carbon sources.

Health & Welfare
Nitrifying biofilms critical for water quality in intensive shrimp RAS
Nitrifying bacteria readily form biofilms on surfaces, and colonization by these important bacteria on the interior walls of RAS production units likely provides an additional source of nitrification.
Innovation & Investment
Estimating biofilter size for RAS systems
Controlling total ammonia-nitrogen (TAN) concentrations is the primary concern when sizing a biofilter for use in a recirculating aquaculture system. Sizing decisions are best based on previous experience with a given biofilter media in a specific biofilter configuration.



