Study suggests potential to increase survival, reduce FCR in biofloc-dominated culture system

In aquatic species, intestinal bacteria are dependent on the external environment and are transient in nature, due to continuous uptake of water, food and associated microorganisms. Probiotics have been shown to change the intestinal microbiota by competing with pathogens for nutrients. Addition of probiotics to feed may therefore contribute to overall health, resulting in increased production.
Biomin produces an aquaculture product line (AquaStar®) formulated to improve larval and grow-out shrimp production or pond water quality. Each product consists of several probiotic bacterial strains, and AquaStar® Growout was designed to be added to feed to enhance grow-out production.
Previous trials with this probiotic in clear water tank system with Pacific white shrimp (Litopenaeus vannamei) in Vietnam resulted in increased growth, survival and immune function. In contrast to clear water systems, biofloc dominated systems contain aggregate particles formed from various components, including bacteria, protozoans, detritus and feed and more closely mimics a pond-based system.
In another study, a combination of this probiotic and AquaStar® Pond resulted in improved survival and FCR in an indoor biofloc-dominated shrimp production system, however the culture medium was contaminated with pathogenic Vibrio. The current trial was initiated to determine the impact of three levels of AquaStar® Growout probiotic (2, 3 or 5 g/kg feed) on shrimp performance in an intensive indoor biofloc-dominated shrimp tank system.
Experimental design
A 12-week study was carried out at Florida Atlantic University’s Harbor Branch Oceanographic Institute (FAU-HBOI) in Ft. Pierce, Fla., in a climate-controlled greenhouse. A 4×4 factorial random block experimental design consisted of sixteen 1-cubic-meter (4-foot diameter) cylindrical tanks fitted with air-driven floc circulation systems filled with salt well water (34 ppt) (Fig. 1).

Sixteen liters of biofloc from the FAU-HBOI Integrated Multi-Trophic Aquaculture (IMTA) ex-situ bioreactor were added to each tank three weeks prior to shrimp addition. One hundred 1.5-gram shrimp were placed into each tank on May 2, 2017. AquaStar® Growout was top dressed onto Zeigler shrimp HI-35 feed (2 mm pellets) at 0, 2, 3 or 5 g/kg (0 percent, 0.2 percent, 0.3 percent, or 0.5 percent).
The powdered probiotic was added to distilled water at a 1:20 dilution rate as per label instructions and dried overnight at 65 degrees-C. Shrimp were initially fed twice a day by hand (weeks one and two), after which feed was fed continuously using belt feeders based on body weight.
Dissolved oxygen (DO), temperature and salinity were measured twice daily and settable solids (SS) were measured once a day using Imhoff cones. Total suspended solids (TSS), alkalinity, total ammonia nitrogen (TAN), nitrites (NO2-N) and nitrates (NO3-N) were measured twice weekly. Sodium bicarbonate was added as needed to maintain alkalinity between 100 and 150 mg/L.
Ten shrimp were removed weekly from one designated tank per treatment and weights recorded. Average survival, final weight, yield, specific growth rate, weekly growth rate and feed conversion ratio were evaluated at harvest. Water was collected weekly from each tank and plated in triplicate on TCBS Vibrio selective agar.
At experimental termination water was additionally plated on marine agar (MA) to determine total bacterial counts and on MacConkey agar (MAC) plate to determine enteric bacteria counts. At harvest the intestinal tract from five shrimp from each tank were collected, pooled, macerated and plated onto TCBS, MA and MAC plates.
Results
Water quality was similar for all treatment groups. Temperature, salinity, pH and dissolved oxygen levels averaged 28.2-28.9 degrees-C, 38 grams/L, 7.6 and 6.6 mg/L, respectively. TAN, NO2-N, NO3-N, and alkalinity averaged 0.05 mg/L, 0.04 mg/L, 4.4-4.7 mg/L and 139-142 mg/L, respectively. Settable solids increased from an average of 8 ml/L to 29 ml/L and TSS increased from 78 to 306 mg/L over the 12-week period.
Production results are shown in Table 1. Although there was a trend towards higher survival (p=0.056), yield (p=0.059), and lower FCR (p=0.060) in the 0.5 percent treatment differences were not significant. Mean survival was 76.5 percent, and highest in the 0.5 percent treatment. Shrimp reached an average of 23.25 grams in 12 weeks, with weekly growth rate averaging 1.5 grams. Mean yield per tank was 1,556 grams, and highest in the 0.5 percent treatment. Mean FCR was 1.39 and was lowest in the 0.5 percent treatment.
Laramore, AquaStar, Table 1
| Parameter | 0% | 0.2% | 0.3% | 0.5% |
|---|---|---|---|---|
| Survival (%) | 73±10 | 67±10 | 81±6 | 85±7 |
| Final weight (grams) | 23.9±2.6 | 24.7±1.3 | 21.9±1.3 | 22.1±3.0 |
| Weekly growth (grams) | 1.9±0.22 | 2.0±0.13 | 1.7±0.11 | 1.7±0.25 |
| Yield (grams/tank) | 1,489±79 | 1,374±325 | 1,599±69 | 1,766±154 |
| FCR | 1.4±0.08 | 1.6±0.43 | 1.3±0.06 | 1.2±0.10 |
Vibrio water column counts began to increase in week four and peaks were noted at weeks 7 and 12. There was a trend towards lower pathogenic (green) Vibrio counts as the amount of probiotic was increased; on average pathogenic Vibrio made up 17 percent of the total Vibrio count in the control, 13 percent in the 0.2 percent and 0.3 percent treatment groups, and 8.5 percent in the 0.5 percent treatment group (Fig. 2). Water samples collected at harvest showed no difference between treatments in total bacterial (MA) or Vibrio counts, however the amount of bacterial growth on MacConkey agar was on average 10x lower (p=0.045) (Fig. 3A).

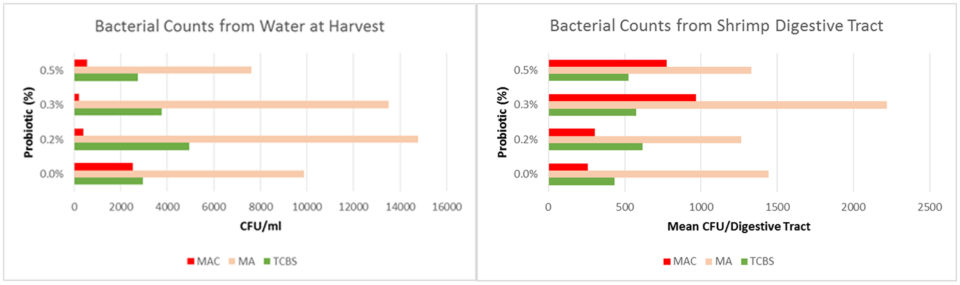
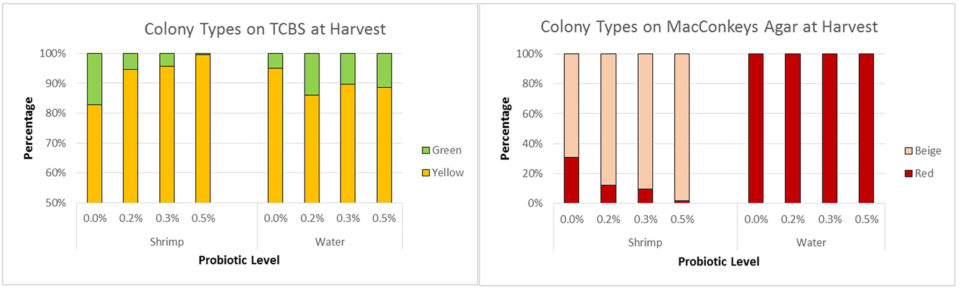
There was no difference in mean bacterial counts from shrimp intestines collected at harvest (Fig. 3B). However, the composition of both Vibrio and enteric bacteria was altered by the probiotic. Shrimp in the control treatment had a higher percentage of pathogenic Vibrio, despite that the percent of pathogenic Vibrio in the water was less (Fig. 4A). Shrimp in probiotic treatments had a lower percentage of beige (non-lactose fermenting) colonies compared to red (lactose fermenting) colonies; no beige colonies were detected in any water samples (Fig. 4B).
Perspectives
Although no significant differences were seen in terms of increased production, bacterial composition differences were noted in both the water and the shrimp intestines. The preponderance of evidence suggests that incorporating this probiotic product, particularly at 0.5 percent, has the potential to positively impact shrimp health and production by increasing survival and thus reducing FCR.
References available from corresponding author.
Authors
-
Susan Laramore, Ph.D.
Corresponding author
Harbor Branch Oceanographic Institute at Florida Atlantic University
Fort Pierce, Florida 34946 USA[117,100,101,46,117,97,102,64,49,111,109,97,114,97,108,115]
-
Tzachi Samocha, Ph.D.
Marine Solutions and Feed Technology, LLC
Spring, Texas USA -
Rui A. Gonçalves
BIOMIN Holding GmbH
Erber Campus 1, 3131 Getzersdorf, Austria -
Jutta Kesselring
BIOMIN Holding GmbH
Erber Campus 1, 3131 Getzersdorf, Austria
Tagged With
Related Posts

Health & Welfare
Ammonia addition enhances microbial flocs in nursery phase for Pacific white shrimp
In a study, “pre-fertilization” in the nursery phase of a biofloc system for shrimp was tested. The objective was to accelerate the biofloc formation to minimize ammonia concentrations, avoiding high peaks during culture.

Health & Welfare
Economic analyses project rising returns for intensive biofloc shrimp systems
In trials raising larger juvenile shrimp than those used previously in indoor super-intensive recirculating raceway systems, the positive effects from increased stocking size, growth rate and survival resulted in a reduced crop duration time.

Aquafeeds
Biofloc consumption by Pacific white shrimp postlarvae
The stable isotopes technique with δ13C and δ15N can be used to determine the relevance of different food sources to shrimp feeding during the pre-nursery phase of Litopenaeus vannamei culture. During this trial, different types of commercial feed, microalgae, Artemia sp. nauplii and bioflocs were used as food sources.

Innovation & Investment
Artemia, the ‘magic powder’ fueling a multi-billion-dollar industry
Artemia, microscopic brine shrimp used as feed in hatcheries, are the unsung heroes of aquaculture. Experts say artemia is still inspiring innovation more than 50 years after initial commercialization. These creatures are much more than Sea-Monkeys.


