Researchers explore solutions to major shrimp disease
The white spot syndrome virus (WSSV) has caused major economic losses to shrimp farmers worldwide since the 1990s. Various field treatments and strategies for controlling WSSV have been tested, showing different degrees of efficacy. Some of these include immuno-stimulants, administration of recombinant viral proteins, manipulating water temperature, DNA vaccines and RNA interference (RNAi).
WSSV has up to 531 putative genes, some of which may be essential for WSSV infection/replication. Most of the WSSV genes have unknown roles in virus infection. Several studies have used RNAi against WSSV genes, encoding structural proteins involved in virion architecture and virus entry. Other studies have assessed the antiviral efficacy of non-structural WSSV proteins.
It is possible the antiviral efficacy of RNAi molecules depends on the targeted genes. Silencing WSSV genes with critical roles in virus replication might show a stronger antiviral effect and thus reduce shrimp mortality.
Study setup
The authors therefore established a study to evaluate the antiviral efficacy of double-stranded (ds)RNA against non-structural WSSV genes orf89 and wsv191 in Pacific white shrimp (Litopenaeus vannamei) and compare it to dsRNA against WSSV genes vp28 and vp26 under experimental conditions.
Juvenile shrimp from a hatchery in Sonora, Mexico, were tested WSSV-negative by polymerase chain reaction (PCR) analyses. The animals were maintained in artificial seawater with a salinity of 25 g/L, temperature of 27 ± 2 degrees-C and continuous aeration. Fifty percent water exchange was done every third day to maintain good water quality.
A WSSV inoculum prepared from naturally infected shrimp from Sinaloa, Mexico, was titrated in vivo. For each tenfold serial dilution (10-2-10-7), five shrimp were intramuscularly inoculated and individually kept in 12-L tanks with artificial seawater.
The shrimp were monitored twice daily for clinical signs of WSSV infection and mortality for 10 days. Infectivity and lethal titers were determined to be 105.6 SID50/mL and 105.6 L.D.50/mL, respectively. SID50 is the shrimp infectious dose that will result in 50 percent infected shrimp. L.D.50 is the single dose that will cause death in 50 percent of a group of test animals.
RNAi against four WSSV genes – vp26, vp28, vp191 and orf89 – was produced as dsRNA using a commercial transcription kit following the manufacturer instructions. Table 1 includes information on the specific primers and standard PCR conditions used to amplify the genes.
Treatments
Groups of 10 WSSV-free L. vannamei were acclimatized for 24 hours in 80-L tanks containing artificial seawater at 25-g/L salinity, a temperature of 25 ± 2 degrees-C and with continuous aeration. Each group was respectively inoculated via 4-µg injections with one of the dsRNAs.
A group was treated with 40 µL dsRNA against bacterial LacZ gene and used as a control. Another group of 10 shrimp was mock treated with 40 µL phosphate-buffered saline and used as a positive control. Three replicates were done per treatment.
After 48 hours, all the treated animals were intramuscularly challenged with a high dose (2,500 SID50 in 50 µL) of WSSV. The shrimp were monitored twice daily for 10 days after the challenge for clinical signs of disease and mortality. Moribund and dead shrimp were recorded and removed from the tanks, with tissue samples taken for WSSV detection.
At the end of the experiment, surviving shrimp were sacrificed, and their gills were used for RNA extraction and WSSV PCR analyses. The antiviral efficacy of the different dsRNAs was assessed using cumulative mortality and number of WSSV-infected shrimp.
Results
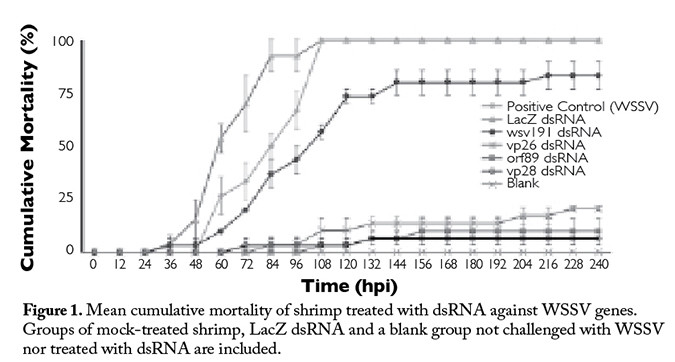
The mock-treated group and shrimp treated with LacZ dsRNA first showed clinical signs of WSSV infection, such as reduced feeding, erratic swimming and/or lethargy at 24 hours post inoculation (hpi). The first mortalities were recorded at 36 hpi. These two groups had 100 percent mortality at 108 hpi (Figure 1), and all animals in these groups were WSSV-positive by PCR (Figure 2).
Shrimp treated with vp28 dsRNA showed first mortalities at 72 hpi and final cumulative mortality of 7 percent. Animals treated with orf89 dsRNA displayed first mortalities at 108 hpi, and their cumulative mortality at the end of the experiments was 10 percent. Shrimp treated with vp26 dsRNA showed first mortalities at 84 hpi and cumulative mortality of 21 percent. Animals treated with wsv191 dsRNA showed first mortalities at 24 hpi and final cumulative mortality of 83 percent (Figure 1).
All dead animals in these treatments were WSSV-positive by PCR. The surviving shrimp did not display clinical signs of WSSV infection and were WSSV-negative by RT-PCR assays (Figure 3).
Antiviral response
A single dsRNA dose of 4 µg/shrimp was enough to trigger an antiviral response against a lethal WSSV challenge. Sequence-specific dsRNA effectively inhibited virus replication and reduced mortality in treated shrimp. At the end of the experiments, most of the animals treated with dsRNA against WSSV genes survived and had low rates of WSSV infection. In contrast, shrimp treated with sequence-independent dsRNA (bacterial LacZ gene) became infected and only underwent a delay in time to mortality.
Shrimp treated with dsRNA against vp28 and vp26 were protected against a highly lethal WSSV challenge. Genes vp28 and vp26 encode structural proteins VP28 and VP26, which have been shown to play important roles in virion structure, virus attachment and infection.
The two dsRNAs against the non-structural WSSV genes orf89 and wsv191 showed that silencing the orf89 gene was effective in reducing virus replication and shrimp mortality in a way similar to that observed with vp28 dsRNA. In contrast, dsRNA against wsv191 had the least antiviral effect of the four sequence-specific dsRNAs tested.
Perspectives
The functions of the two non-structural proteins encoded by these WSSV genes may help explain the differences in antiviral activity. Gene orf89 is expressed early during virus replication and encodes a viral protein that has a negative regulatory function. This gene may have an essential early role during virus infection/replication, and therefore its silencing greatly affected the outcome of WSSV infection. In contrast, gene wsv191 probably is not an essential gene for WSSV replication, despite the fact it may encode a non-specific endonuclease with both DNAse and RNAse activities. Its silencing was not significant in inhibiting virus replication.
| Primer Name | Sequence (5' > 3') | PCR Product Size (bp) |
|---|---|---|
| orf89F1 orf89R1 | AGGACCCGATCGCTTACTTTGA CAAGAAACCGGGAGGGATTTTC | 483 |
| wsv191F1 wsv191R1 | AAGTGGGTGCGCAACAAAATA TGTAGAGGGCATGAGGGATAG | 451 |
| vp28F3 vp28R3 | ATGGATCTTTCTTTCAC TTACTCGGTCTCAGTGC | 615 |
| vp26F4 vp26R4 | ATCCAACCAACACGTAAAGG GGAAAATCTACATCTGTTGTGC | 580 |
| LacZF2 LacZR2 | ACCAGAAGCGGTGCCGGAAA CCACAGCGGATGGTTCGGAT | 1,012 |
| B-ActinF2 B-ActinR2 | GAAGTAGCCGCCCTGGTTG CGGTTAGCCTTGGGGTTGAG | 416 |
| BLOCK-iT.T7 | GATGACTCGTAATACGACTCACTA |
Authors
-
C. M. Escobedo-Bonilla
Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional
Instituto Politécnico Nacional
Unidad Sinaloa
Blvd. Juan de Dios Batiz Paredes 250
Colonia San Joachin, Guasave
Sinaloa 81020 México[109,111,99,46,111,111,104,97,121,64,120,109,111,100,101,98,111,99,115,101,95,114,97,115,101,99]
-
S. Vega-Peña
Universidad Autónoma de Baja California Sur
La Paz, Baja California Sur, México -
C. H. Mejía-Ruíz
Centro de Investigaciones Biológicas del Noroeste
La Paz, Baja California Sur, México
Related Posts

Health & Welfare
Genetic variation for resistance to WSS, AHPND in Pacific white shrimp
Selection for disease resistance has been used in breeding farm animals and can be a viable option to deal with white spot syndrome and acute hepatopancreatic necrosis disease in commercial shrimp culture. In trials, heritability for AHPND resistance was low, while that for WSS was moderate.
Health & Welfare
On-site diagnostic kit identifies WSSV in shrimp
Loop mediated isothermal amplification (LAMP) is a highly specific technique that can amplify DNA in isothermal conditions. The kit can amplify the target gene of the pathogen using a single temperature and give results in one hour.

Health & Welfare
Study: Inbreeding affects body weight, not survival, in white shrimp
Inbreeding commonly causes a decrease in the mean values of traits of productive interest in aquaculture. Results of a study, however, showed that inbreeding had no effect on grout survival of Pacific white shrimp from 65 to 130 days of age.

Health & Welfare
The history and future of the Aquaculture Pathology Laboratory
The University of Arizona’s Aquaculture Pathology Laboratory has significantly contributed to the expansion of the shrimp farming for three decades.


