Varied staining methods identify pathogens
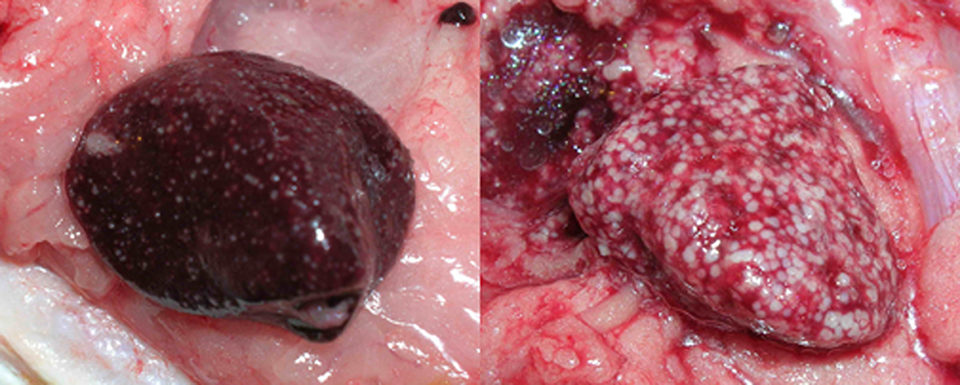
One of the most familiar aspects of health maintenance in cultured fish species is that of chronic disease characterized by the presence of white or tan nodules in one or many organs. Such nodules are due to chronic inflammation in tissue in which macrophages, a type of white blood cell, gather into populations known as granulomas.
Granulomas have infectious and non-infectious causes, can vary in appearance and have a natural history that is intimately involved with immune function. In short, they are a means by which a body isolates and eliminates undesirable material, but the degree to which granulomas are present is in part determined by the body’s success in controlling offending agents.
Granulomatous inflammation
It is common for healthy fish to have one to several 1- to 2-mm-wide granulomas in their internal organs. These granulomas are often due to parasites or non-infectious material.
However, numerous granulomas in diseased fish are characteristic of infections by bacteria such as mycobacteria, nocardiae and francisellae. These granulomas may be seen as spherical, often melanized structures in wet mounts of organ squash preps.
It is advisable to examine sections of fixed tissues stained with hematoxylin and eosin by light microscopy in any case of granulomatous disease to verify microbiological results and determine if more than one pathogen is present.
Mycobacteria
Mycobacteria are arguably the most common and well-known cause of granulomatous disease in fish. They can affect both fresh- and saltwater species, and all fish should be considered susceptible.
Mycobacteria are gram-positive, acid-fast bacteria that form pleomorphic rods, filaments or cocci. Until the last decade, only a handful of species (M. chelonae and M. fortuitum, for example) were identified as fish pathogens, but this list has expanded as methodology for speciation improved. Mycobacterium marinum, a frequent isolate from diseased fish, is a fast-growing species that shows growth in specific solid media in seven to 14 days, while other species can take months for colony growth.
It is important to understand that if cultures from diseased tissues are not obtained, mycobacteria can not be ruled out as a causative pathogen. The isolation of mycobacteria and presence of granulomas and acid-fast bacteria in lesions may not be well correlated. It is prudent to save frozen and fixed tissues for use in polymerase chain reaction testing and histopathology to solidify a diagnosis.
Disease signs
Diseased fish show a variety of external signs that range from ulcers, exophthalmia and emaciation to lethargy, visible granulomas or no signs at all. The course of disease is typically chronic, although acute mortalities have been reported.
Internally, gray, tan or white foci are present in any organ, but typically the spleen, liver or kidneys. The foci may coalesce, leading to partial or total replacement of the organ by inflammation.
Microscopically, prototypical granulomas composed of macrophage aggregates rimmed by lymphocytes and fibrocytes can be seen in diseased fish. Granulomas, which can have necrotic cores, typically mature through characteristic stages with overall progressive fibrosis. The presence of identifiable bacteria is highly variable, and in many cases, special stains are used to visualize them.
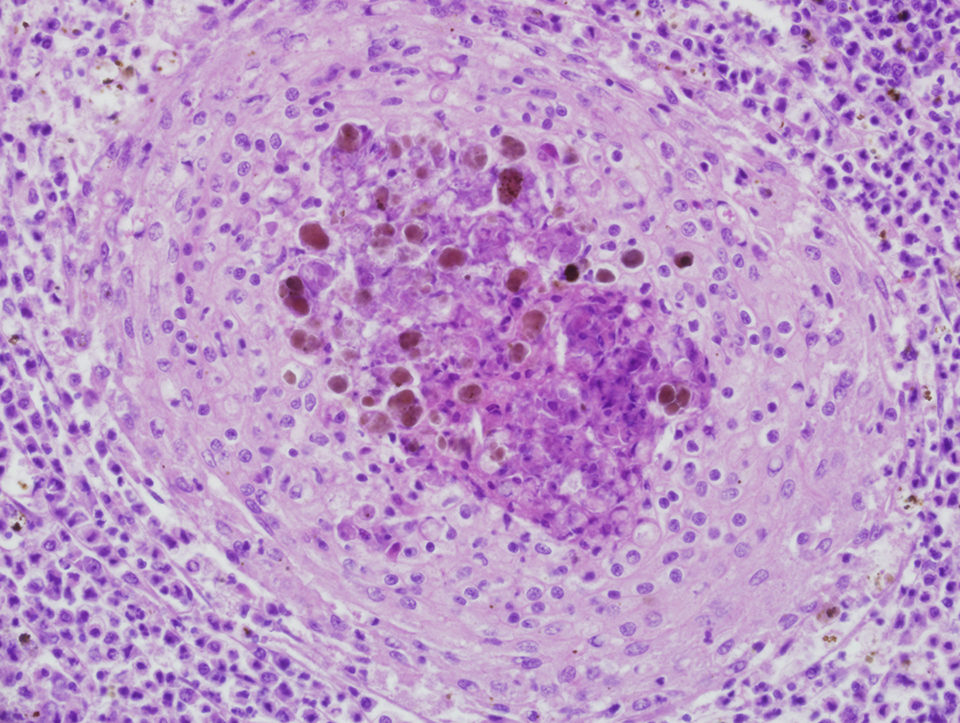
Tissue stains
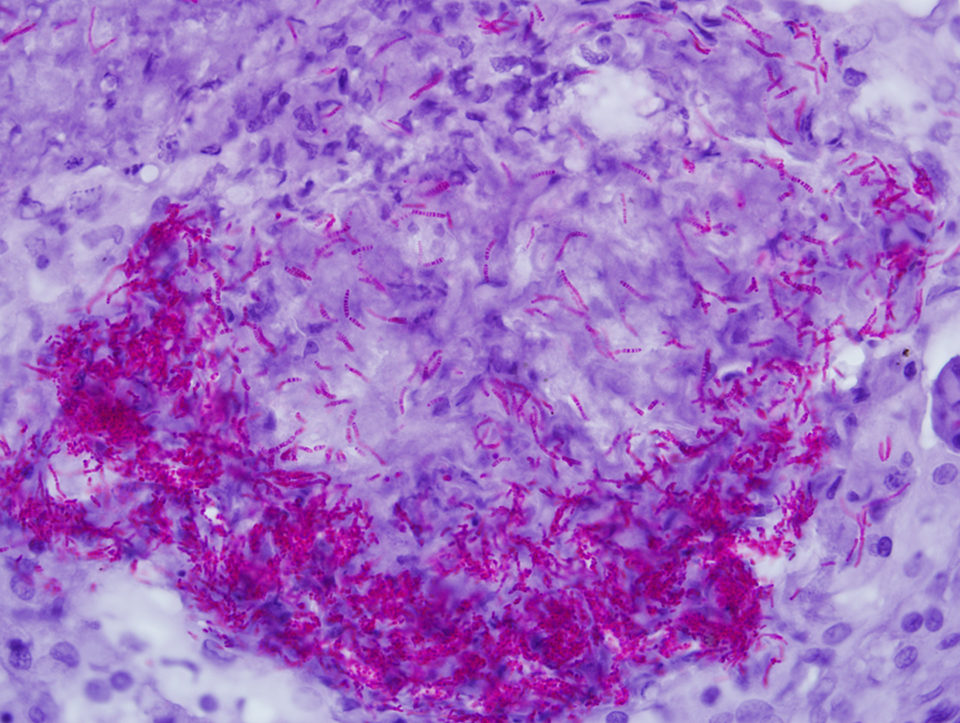
The best known, often used and most convenient tissue stain for mycobacteria is the “acid-fast” stain, but fluorescent and immune-based tissue stains are also available. Acid fastness in the mycobacterial sense is the ability of bacteria to resist decolorization by 3 percent acid alcohol. It is important to recognize that this stain is not specific, since nocardiae and rhodococci can also be acid-fast. Further, acid fastness is not sensitive. Less than 1 percent of mycobacteria can be acid fast in some cases.
The degree of acid fastness can vary widely due to the fixation method, staining recipe, presence of mycobacteria with intact cell walls, type and amount of mycolic acid in bacterial walls and growth stage of the bacteria. It is not rare to find few to no acid-fast bacteria in tissue slides from cases of mycobacteriosis.
The Ziehl-Neelsen stain for acid fastness is perhaps the most commonly used and reported technique for diagnosis of mycobacteria in fixed tissues. However, this staining procedure is not standardized and has many variations, so methodologies should be specifically reported when using the stain.
Many have advised the use of periodic acid as an oxidizing agent to improve acid fastness in tissue sections, yet such a treatment alters the nature of bacteria in a mycobacteria-specific sense. While periodic acid improves bacterial staining with acid-fast stains, it also makes the Ziehl-Neelsen (Z.N.) stain useless for the diagnosis of mycobacteria and nocardiae in disease outbreaks.
Nocardiae
Nocardiae are gram-positive, weakly acid-fast, branching rod bacteria that affect both fresh- and saltwater species. Nocardia asteroides, N. seriolae and N. salmonicida are the most common isolates. They can take up to three weeks to grow in solid media at 25 degrees-C. The presence of branching aerial hyphae in culture colonies is characteristic.
The clinical signs of disease from nocardiae are similar to mycobacteriosis. Nocardiae are often said to be acid-fast, but this depends heavily on the staining method applied. Modified Z.N. stains use mineral acid rather than acid alcohol, which may be more effective. Additionally, 3 percent acid alcohol is the standard Z.N. decolorizer, which will often decolorize nocardiae. If 1 percent acid alcohol is used, as in some commercial Z.N. stains, nocardiae retain the stain.
Francisellae
Francisellae have been known as mammalian pathogens for nearly a century. However, only recently have they become identified with significant disease in farmed fish.
Francisellae have been confused with piscirickettsia-like organisms in fish disease outbreaks, and distinguishing between the two is problematic. Francisella noatunensis and F. asiatica have been identified as emerging pathogens in a growing list of fish, including cod, tilapia, grunt, salmon, cichlids and hybrid striped bass.
They are gram-negative coccobacilli that infect macrophages, causing characteristic granulomas in many tissues. These bacteria won’t grow in simple media and are often difficult to isolate, even from obviously diseased specimens. As they grow slowly – taking five to seven days to show visible colonies – overgrowth of the francisellae by contaminating bacteria is a problem. Selective media is recommended for primary isolation. The selective medium the authors use is produced by adding 50 µg/mL ampicillin and 100 units/mL polymyxin B to cysteine heart agar fortified with bovine hemoglobin.
F. asiatica, which causes severe and widespread granulomatous disease in farmed tilapia, originally presented as a necrotizing myositis that caused fillet condemnation at processing plants. Granulomatous disease primarily affects the head kidney, spleen and liver of the fish. Disease outbreaks are affected greatly by water temperatures below 28 degrees-C, and the progression of lesions is relatively rapid.
Within macrophages, 0.5- to 1- µ-wide cocco bacilli are present in tremendous numbers in many cases. Bacteria are not acid fast sense and are most easily seen with Giemsa stains, but standard hematoxylin and eosin stains also work well.
(Editor’s Note: This article was originally published in the July/August 2011 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Wes A. Baumgartner, DVM, Dipl. ACVP
Louisiana State University School of Veterinary Medicine
Department of Pathobiological Sciences
Skip Bertman Drive
Louisiana State University
Baton Rouge, Louisiana 70803 USA[117,100,101,46,117,115,108,46,115,114,101,103,105,116,64,49,103,109,117,97,98,119]
-
John Hawke, Ph.D.
Louisiana State University School of Veterinary Medicine
Department of Pathobiological Sciences
Skip Bertman Drive
Louisiana State University
Baton Rouge, Louisiana 70803 USA
Related Posts

Health & Welfare
Off-flavors in salmonids raised in RAS
The presence of compounds such as geosmin and 2-methylisoborneol (MIB) in recirculating aquaculture systems (RAS) can result in earthy or musty off-flavors in salmonids raised in the systems.

Responsibility
Addressing safety in Latin America’s tilapia supply chain
Over the last decade, the experience gained by many tilapia farmers combined with proficient programs implemented by local governments have significantly improved tilapia production in various Latin American countries like Colombia, Mexico, Ecuador and other important tilapia producers in the region.

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.



